Recommendations on Standards for the Design of Medical Diagnostic Equipment for Adults with Disabilities, Advisory Committee Final Report
Appendix B: Supporting Documents
Supporting Documents for the Medical Diagnostic Equipment Accessibility Standards Advisory Committee Report
A number of key documents generated in support of the Medical Diagnostic Equipment Accessibility Standards Advisory Committee work were utilized in the Committee’s final report “Advancing Equal Access to Diagnostic Services: Recommendations on Standards for the Design of Medical Diagnostic Equipment for Adults with Disabilities”. These have been collected together and are listed following. Each is linked to this document.
Supporting Documents Files:
- Subcommittee Report - Exam Table and Chairs
- Subcommittee Report - Imaging Equipment
- Subcommittee Report - Mammography
- Subcommittee Report - Stretchers
- Subcommittee Report - Weight Scales
- IDeA Center Memo – Transfer Surface Dimensions
- IDeA Center Memo - Mammography Knee Clearance Depth and Footrest Clearance
- IDeA Center Memo - Wheelbase Measurements
Examination Tables and Chairs Subcommittee Report (including height position discussions as requested by full committee)
May 28, 2013
Introduction
The Tables & Chairs Subcommittee met six times via conference call. We began our discussion by reiterating that the intent of the proposed standard is to provide for independent access to and use of diagnostic equipment.
First, we discussed in length the definitions/differences between examination tables and chairs to understand the medical equipment we are discussing. Subcommittee members felt that the definitions should be based on how the device is defined by manufacturer as follows:
Manufacturers design examination tables with primary functions of supporting patients in a prone position, side-lying position, or both.
Manufacturers design examination chairs with primary functions of supporting patients in a seated or supine position.
Summary of Recommendations
Transfer Surface Size – M302.2.2 Seated Position
The issue of seat depth was discussed and decided during the full committee meetings as follows: depth of 17 inches minimum. The Subcommittee discussed the width and agreed on a 21 inch minimum width based on anthropometric data and industry human factors experience. In addition, examination chairs that cannot be entered on the foot end, such as dental chairs and podiatry chairs, the subcommittee members agreed that the 17 inch minimum depth and 21 inch minimum width be located on both sides of the chair to allow for a left or right transfer (see examples below).
Transfer Surface Size – M301.2.2 Supine, Prone, or Side-Lying Position
The issues were discussed and decided in the full committee meeting as follows: depth of 17 inches minimum and width of 28 inches minimum.
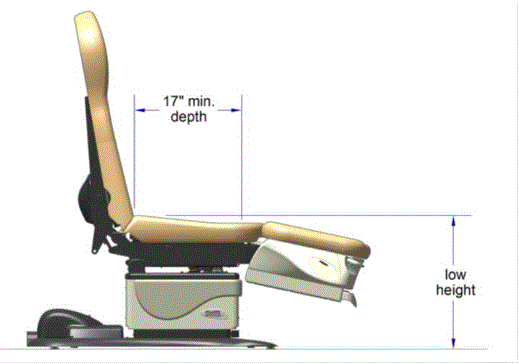
Podiatry Chair, Side View
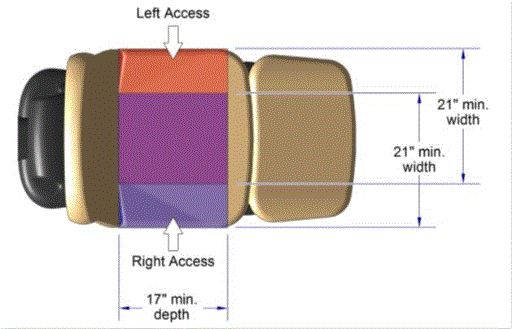
Podiatry Chair, Top View
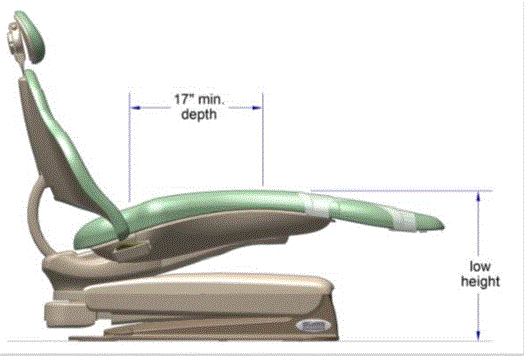
Dental Chair, Side View
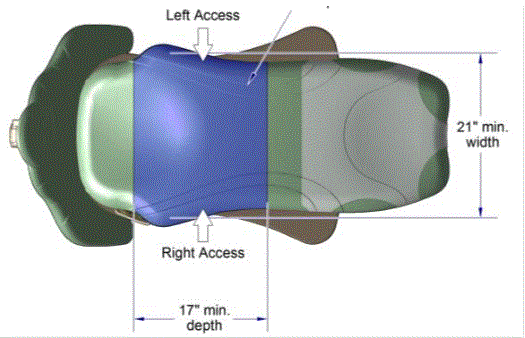
Dental Chair, Top View
Height – M301.2.1 & M302.2.1
The subcommittee discussed the increments for adjustability and decided that infinite adjustability is the appropriate standard as both electronic and manual pump chairs have ability to stop at any position.
The discussion for the low end of the minimum range consumed a majority of the Subcommittee’s time. The final recommendation of the subcommittee to the full committee was a recommendation of 19 inches with a "best practice" standard of 17 inches. Upon consideration of the subcommittee’s final recommendation, the full committee was unable to come to a consensus around any specific low height requirement, nor the “best practice” recommendation. Therefore, the report presents each of the arguments in favor of the proposed low height measurements – 19 inches, 18 inches, and 17 inches:
17 Inch Low Height
A portion of the Committee maintained that a height range of 17 inches - 25 inches would accommodate a significant portion of people with disabilities. Data provided to the Committee and expert advice of medical practitioners led many to conclude that a lower height of 17 inches is essential to ensure safe transfers by patients with disabilities and thus, accomplishes accessibility for the greatest number of people.
Studies of Wheeled Mobility Devices and Transferring Abilities
Accessible medical equipment needs to facilitate safe transfers that accommodate the largest possible portion of people with disabilities, including people who use wheeled mobility devices. The safest and most easily accessible transfers are those with no or very little horizontal and vertical distance between the seat of the wheelchair and the transfer surface. Specifically, transferring to a higher surface applies greater exertion of the upper limbs.1
A study of wheeled mobility devices, including manual wheelchairs, power wheelchairs, and scooters examined the seat height of 495 users. The height was measured as the vertical distance from the floor to the lowest point of the seating surface of the mobility device, while the occupant was seated in the device. Thus, the surface of the mobility device was in a compressed state. The study noted that a range of 17 inches -25 inches accommodates the vast majority of wheeled mobility device users, while continuing to exclude 6% of manual wheelchair users whose devices are lower than 17 inches. Increasing the low end to 19” height excludes many users, specifically over 30% of female manual chair users and over 15% of male manual chair users.2
There is limited information on the ability of people with disabilities to transfer to a height different from the height of their wheeled mobility device. The Impact of Transfer Setup on the Performance of Independent Transfers: Final Report provides an analysis of the effect of height, horizontal gap, placement of armrests, and placement of grab bars on a person’s ability to transfer. The study noted that 86% of wheeled mobility device users could transfer to heights that were 2 inches above and below the height of their wheeled mobility device. However, this study was not representative of the diversity of wheeled mobility device users. Individuals were explicitly excluded from the study if they had significant upper extremity pain or injury that affects the ability to perform transfers, or had an active or recent history of pressure sores. Furthermore, the vast majority of subjects in the study were men.3 Numerous research studies as well as anecdotal reports from people with a variety of mobility disabilities (spinal cord injury, cerebral palsy, polio, traumatic brain injury, etc.) have detailed and reinforced that fact that people who live with disability experience a greater prevalence of and earlier onset of age related conditions such as arthritis, pain contractures, weakness, deconditioning, and shoulder injuries etc.4
Data Deficiencies
Advocates on the committee, representing the interests of people with disabilities, strongly stated that a 17” height is essential to accommodate the largest segment of people with disabilities. While general information can be found regarding average height of wheeled mobility devices, and people’s ability to transfer, data is not available regarding the needs of people of short stature and people with mobility disabilities who do not use wheeled mobility devices. The promulgation of standards for accessibility is required to take into account all people with disabilities, not just those who use wheeled mobility devices. In the absence of additional data, being inclusive versus exclusive is the most responsible approach in the development of standards.
Expert Advice of Medical Practitioners
Numerous practitioners commented on the importance of the availability of a lower transfer surface. Nüket J. Curran, PT, Director, Quality & Risk Management at UPMC Centers for Rehab Services, noted the importance of patient safety, staff safety, and ease of use. She stated that a 14 inch high transfer surface on tables and chairs would be ideal to facilitate the safest transfers, noting that 17 inches can be too high for some people. Ms. Lauren Snowdon, PT, DPT, Clinical Manager at the Kessler Institute for Rehabilitation, provided in depth analysis of the transferring abilities of wheeled mobility device users and the ideal height of transfer surfaces. Based on her experience that the general range of customized manual wheelchair height is 15.5 inches- 19.5 inches and that power wheelchairs range from 16.5”- 22” high, she stated that the option of a 17 inch high surface would be preferable to a 19 inch height. A 17 inch height allows for safer, easier transfers of patients whose wheelchair height is at the low end of those ranges. Other practitioners stated that the current height option of 18” was satisfactory and facilitated safe transfer for most patients. However, within this group, there was also a general consensus that a 17 inch height would provide greater accessibility, and enhance the safety of transfers for some patients. Given the importance of safe transfers and height ranges of wheeled mobility devices, a low height of 17 inches is considered by some to be a compromise solution.
The Limits of Utilizing a Cost-Benefit Analysis
Many advocates strongly cautioned against attempting a strict cost/benefit analysis when ample data is unavailable. To our knowledge there are no studies that provide a fully comprehensive analysis of the effects of the height of a transfer surface on people with various disabilities. Additionally, every time a patient with a disability is denied access to health care due to an inability to access equipment, the cost is often far more than traveling to a different health care facility. Delayed diagnosis and treatment translates into higher HEALTH CARE costs due to the need for more extensive and expensive treatment. Furthermore, the cost of the systemic denial of health care to these individuals can be life threatening.
There is additional risk to patients when medical practitioners attempt to manually transfer patients to diagnostic equipment. Risk of injuries due to being dropped, as well as skin shear and shoulder injuries can occur when the transfer surface cannot be adjusted low enough to accommodate a straight transfer for individual wheeled mobility devices of varying heights. The cost of medical practitioner injuries while performing these types of tasks has been widely documented and should also be considered.
While, there are always practical limitations on accommodating every individual, in the case of access to a service as essential as health care, advocates strongly urge the Access Board to develop standards that are not only practical to industry interests, but will guarantee access to the vast majority of people with disabilities.
Notes
1. The Impact of Transfer Setup on the Performance of Independent Transfers: Final Report. Presentation to US Access Board. Washington, DC. 2011
2. D’Souza, Clive and Edward Steinfeld, IDeA Center. Analysis of Seat Height for Wheeled Mobility Devices. 2011.
3. The Impact of Transfer Setup on the Performance of Independent Transfers: Final Report. Presentation to US Access Board. Washington, DC. 2011
4. Jensen, M.P., Molton, I.R., Groah, S.L., Campbell, M.L., Charlifue, S., Chiodo, A., Forchheimer, M., Krause, J.S., & Tate, D. (2011). Secondary Health Conditions in Individuals Aging with SCI: Terminology, Concepts, and Analytic Approaches. Spinal Cord, 50(5): 373-378.
Groah, S.L., Charlifue, S., Tate, D., Jensen, M.P., Molton, I.R., Forchheimer, M., Krause, J.S., Lammertse, D.P., & Campbell, M. (2012). Spinal Cord Injury and Aging: Challenges and Recommendations for Future Research. American Journal of Physical Medicine & Rehabilitation, 91(1): 80. doi: 10.1097/PHM.0b013e31821f70bc. Available from: http://journals.lww.com/ajpmr/Abstract/2012/01000/Spinal_Cord_Injury_and_Aging__Challenges_and.10.aspx. Accessed December 18, 2012.
Turk M. Secondary conditions and disability. In: Field MJ, Jette AM, Martin L (eds). Workshop on disability in America. A new look. Summary and background papers. Board on Health Sciences Policy, Institute of Medicine of the National Academies, The National Academies Press: Washington DC, 2006, pp. 185–193.
Kemp, B.J., & Mosqueda, L. (Eds.) (2004). Aging with a Disability: What the Clinician Needs to Know. Baltimore, MD: Johns Hopkins University Press.
Kailes, J. (2000). Health, Wellness and Aging with Disability, KAILES - Publications, http://www.jik.com/resource.html, jik@pacbell.net This email address is being protected from spambots. You need JavaScript enabled to view it. .
Kailes, J. (1995). "Midlife Cripdom: Getting Fewer Miles per Gallon?" The Disability Rag 16(4).
Kailes, J. (2001). Aging with Disability - Good News and Bad News. Western U-View. XX: 17.
18 Inch Low Height
As our discussions progressed on the low height, whether it should be 17 inches, 18 inches or 19 inches; a compromise position of 18 inches was suggested. Some individuals indicated that they would be willing to compromise, but virtually all of the subcommittee members indicated that the 17 inch low height was the most inclusive and reached a broader range of individuals using wheeled mobility devices.
During a Subcommittee conference call, Dr. Edward Steinfeld, the principal author of the Analysis of Seat Height for Wheeled Mobility Devices, stated 17 inches is the best practice as it accommodates approximately 94% of individuals using wheeled mobility devices according to their research (see above). When asked about the 18 inch as the low height, Dr. Steinfeld stated it could be a reasonable compromise since the group most affected by increasing the height would be manual wheelchair users who could accommodate a 1 inch change in vertical clearance during transfer. He also stated there is limited data on the effect of a level change as the distance (gap) between the wheeled mobility device and the piece of medical equipment increased and cautioned against speculating that height differences between the wheeled mobility device and transfer surface would be acceptable to all users.
Finally, a majority of committee members proposed a range for the low height of 17 to 19 inches rather than establishing an absolute minimum. An example of providing a range is the pool seat lift accessibility standard per ADA and ABA 2004 Guidelines Section 1009.2.4, which clearly establishes that, even with adjustable devices, a range for the highest (or in our case lowest) position is suitable and appropriate. Thus, the standard could be stated as follows: the transfer surface must lower to at least 17 inches to 19 inches and infinitely adjust to at least 25 inches above the finished floor.
19 Inch Low Height
Although the subcommittee’s conversations were complex and far-reaching, the following points summarize the factors that lead to the selection of the 19 inch height recommendation:
-
Ensures that such equipment is accessible to, and usable by, individuals with accessibility needs and will allow independent entry to, use of, and exit from the equipment by those individuals
-
Carefully balances the costs to hospitals, physicians, and other health care providers of replacing or modifying existing equipment together with manufacturer costs of redesigning equipment
-
Provides for the least cost to health care system of any option considered by the subcommittee
-
Results in the most rapid rate of adoption of accessible equipment by health care providers and the benefits of that equipment to individuals with accessibility needs, particularly those who use wheeled mobility devices.
The memorandum explains why a requirement for a 19 inch lower adjustable height for tables and chairs is the most appropriate standard for the initial implementation of section 4203 of the Patient Protection and Affordable Care Act.
Current Situation: the Vast Majority of Examination and Procedure Tables are 32 Inches High
In the United States, approximately 82 percent5 of physicians, hospitals and other health care providers use examination and procedures tables with a 32-inch fixed height, as shown in Figure 1. Industry commonly refers to these tables as "box tables.” These tables provide an often-insurmountable barrier to health care for people with accessibility needs. Since 2001, the number of adjustable-height tables has steadily increased, but continue to represent a minority of examination tables in the United States.
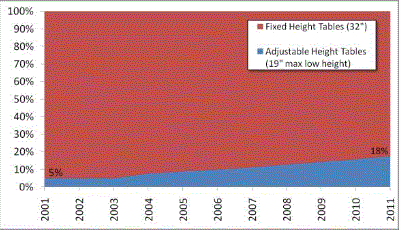
Figure 1: Percentage of fixed height versus adjustable height medical tables
One of the primary objectives of the U.S. Access Board’s requirements should be to accelerate the growing trend of heath care providers to purchase adjustable height tables, which will reduce the number of fixed, 32 inch high inaccessible tables.
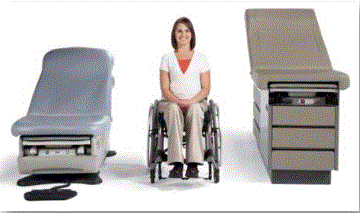
Figure 2: Illustration of the height difference between a fixed height and adjustable height medical table
Implications of a 19 Inch Minimum Standard for the Highest Point in the Lowest Adjustable Position
This memorandum presents the factors that support a minimum standard of 19 inches as the highest point on the transfer surface in a table or chair’s lowest adjustable position. However, shorthand references to 19 inches as the minimum standard as used in this memorandum should not be equated with a 19-inch transfer surface height, for two major reasons. First, as depicted in Figure 3, adjustable tables currently on the market generally feature contoured bolsters that provide greater security once an individual is seated or lying on the table or chair. A 19-inch standard means that any bolsters fit within the highest point standard, thereby making the front edge of the table/chair lower than the bolsters (by about ¾” based on currently marketed bolsters, or about 18 inches compressed at the transfer surface).
Second, as a minimum standard, establishing a 19-inch highest point standard does not mean that all newly manufactured tables and chairs will necessarily be fixed at a 19-inch height. Unlike fixed transfer surfaces such as toilets or non-adjustable tables, there is no reason to standardize at a single height based on broadest usability. In a marketplace of adjustable tables and chairs, increased range of adjustability will be advantageous to patients and caregivers alike. It is not unreasonable to expect that table and chair manufacturers will seek to compete by offering products with greater degrees of adjustability.
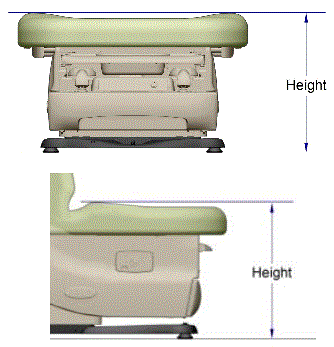
Figure 3. Illustration of measurement height at bolsters relative to lower transfer surface
Alignment of 19 Inch Recommendation with Access Board Proposed Rulemaking
The subcommittee’s recommendation of a lower adjustable height of 19 inches maximum is consistent with the U.S. Access Board’s proposed rule and supported by public comments. In its proposed rule, the Access Board proposed that the “height of the transfer surface during patient transfer shall be 17 inches (430 mm) minimum and 19 inches (485 mm) maximum measured from the floor to the top of the transfer surface” for both examination tables and chairs.6 The Access Board based its proposal, “on provisions in the 2004 ADA and ABA Accessibility Guidelines for architectural features that involve transfers (e.g., toilet seats, shower seats, dressing benches).”7 In addition, the Access Board recommended, “Where patient support surfaces are contoured or upholstered for patient comfort or to support patient positioning during diagnostic procedures, the height of the transfer surface measured from the floor may vary across the transfer surface. The highest and lowest points of the transfer surface on such equipment would have to be within the specified dimensions.”8 The Access Board proposed that the measurement should be taken from the “floor to the top of the upholstery under static conditions, without compression or deflection in the transfer surface ….”9
The Access Board’s proposal also explained that it is considering requiring in the final standards that the height of transfer surfaces be adjustable from 17 inches minimum to 25 inches maximum during patient transfer. In support of the alternative proposal, it cites ANSI/AAMI HE7510 and the Wheeled Mobility Anthropometry Project.11 The results of this study recommended adjustable heights, with an increased maximum height above 19 inches, be provided in order to better accommodate users of powered wheelchairs and scooters. During the committee hearings, the manufacturers accepted that this would be appropriate and offered 19-inch to 25-inch adjustable height through powered tables.
The subcommittee’s recommendation appropriately balances the two proposed alternatives included in the Access Board’s proposed rule. Therefore, the Access Board should adopt the subcommittee’s recommendation provider for continuous adjustability of the height of the transfer surface between 19 and 25 inches.
A Minimum Highest Point standard of 19 Inches is Consistent with Existing Accessibility standards
Current accessibility standards and regulations generally consider a transfer surface height of 19 inches accessible. For example:
Nineteen-inch pool lift seats are accessible:
1009.2.4 Seat Height. The height of the lift seat shall be designed to allow a stop at 16 inches (405 mm) minimum to 19 inches (485 mm) maximum measured from the deck to the top of the seat surface when in the raised (load) position.
Nineteen-inch water closet and toilet seats are accessible:
604.4 Height. The height of water closet seats shall be 17 inches (430 mm) minimum and 19 inches (485 mm) maximum above the floor, measured to the top of the seat. Seats shall not be sprung to return to a lifted position.12
Likewise, 19 inch high benches are accessible:
903.5 Height. The top of the bench seat shall be 17 inches (430 mm) minimum and 19 inches (485 mm) maximum above the floor, measured to the top of the seat.13
Nineteen-inch high bathtub seats are accessible:
610.2 Bathtub Seats. The height of bathtub seats shall be 17 inches (430 mm) minimum and 19 inches (485 mm) maximum above the bathroom floor, measured to the top of the seat. Removable in-tub seats shall be 15 inches (380 mm) minimum and 16 inches (405 mm) maximum in depth. Removable in-tub seats shall be capable of secure placement. Permanent seats shall be 15 inches (380 mm) minimum in depth and shall extend from the back wall to or beyond the outer edge of the bathtub. Permanent seats shall be positioned at the head end of the bathtub.14
Nineteen-inch high shower compartment seats are accessible:
610.3 Shower Compartment Seats. The height of shower compartment seats shall be 17 inches (430 mm) minimum and 19 inches (485 mm) maximum above the bathroom floor, measured to the top of the seat. In transfer-type and alternate roll-in-type showers, the seat shall extend along the seat wall to a point within 3 inches (75 mm) of the compartment entry. In standard roll-in-type showers, the seat shall extend from the control wall to a point within 3 inches (75 mm) of the compartment entry. Seats shall comply with Section 610.3.1 or 610.3.2.15
Nineteen-inch high amusement park rides are accessible:
1102.5.2 Transfer Height. The height of amusement ride seats designed for transfer shall be 14 inches (355 mm) minimum and 24 inches (610 mm) maximum measured from the surface of the load and unload area.16
As mentioned above, a minimum standard of 19 inches at the highest point of a table or chair is not the same thing as the transfer surface height experienced by patients because the front edge of currently available tables is lower than the highest measured point. However, the fact that a nineteen-inch height is so widely accepted by existing accessibility standards for various types of benches and chairs strongly commends maintaining the standard for medical diagnostic equipment as the minimum standard for accessible examination tables and chairs. This is particularly the case because of the manner in which medical diagnostic equipment is used. Unlike toilets, bench seats, and bathtub seats, where usability may require constant contact of one’s feet with the floor for stability or require free use of one’s hands, in most cases medical examination tables and chairs will be raised substantially off of the floor to accommodate caregiver access to patients. Additional requirements of the proposed standards for arm supports provide additional security once an individual has made a successful transfer.
An Increasing Number of Health Care Providers are Transitioning to Adjustable Height Tables
In 2012, approximately 25 percent of examination tables sold in the U.S. are at about a 19-inch low height.17 This is a significant increase over the 17 percent of adjustable height tables sold in 2005. While manufacturers of tables and chairs understand that lower heights may be desirable, no manufacturer has been able to design and produce an examination table or chair that can reach a height lower than 19 inches uncompressed at the highest point on the table or chair. It is unclear when such a table or chair could be available.
If adopted by the Access Board, a recommendation of 19-inch maximum height will build on the growing percentage of providers voluntarily purchasing accessible, adjustable height tables, at 25 percent today.18 Conversely, if a new standard lower than 19 inches is established, thereby deeming all current adjustable tables inaccessible, the entire U.S. health system will be forced to begin at 0 percent accessible.
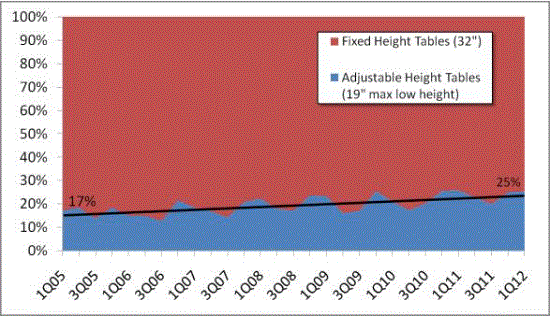
Figure 4: Sales percentages of fixed height versus manual medical tables
Available Data do not Support Departing from the Currently Accepted Standard of 19 Inch Transfer Surface Height
The Medical Diagnostic Equipment Technical Advisory Committee appropriately tried to determine what the optimal accessible transfer surface height is based on available data. In particular, the Advisory Committee spent a great deal of time discussing the Wheeled Mobility Anthropometry Project.19 In that study, which the U.S. Access Board commissioned, Dr. Steinfeld served as the lead investigator. The Wheeled Mobility Anthropometry project did not study optimal transfer surface heights or the ability of wheeled mobility device users to transfer independently from their mobility device onto an examination table or chair. Instead, the study measured the physical characteristics of people who use wheeled mobility devices and some of the characteristics of those devices.
In evaluating data about seat height, we must be sure to take into account the relevant transfer height from the wheelchair. Dr. Steinfeld’s study measured the rear compressed seat height of the wheeled mobility device with the user seated in it. For transfer purposes, however, the most relevant height is the wheelchair front edge. Unfortunately, Dr. Steinfeld did not measure the height of those same users' front wheelchair edges.
There is a recognized international standard defining various measurements of wheelchairs: ISO 7176-7:1998 Wheelchairs — Part 7: Measurement of seating and wheel dimensions. Figures 6 and 7 below are selected screenshots from this international standard. These illustrations identify several key measurements. The most important illustrations for present purposes are "seat plane angle," "effective seat depth," and "seat surface height at the front edge." The standard focuses on measurement procedure so it does not prove any actual measurements. However, as the images below make clear, the seat reference plane and effective seat depth will dictate the difference between the seat surface height at the front edge and the height of the seat at the rear of the wheelchair. Note further that wheelchair height measurements generally do not take into account the height of the cushion, which the consumer will need to clear at the front edge to enable a successful transfer.
In addition, section 4 of the Paralyzed Veterans of America’s Guide to Wheelchair Selection20 also illustrates the distinction between the following heights:
…the seat surface height at the front edge (which excludes the effect of a seat cushion, typically measuring 2-4" in depth), the seat height at the rear of the seated surface, and the relevant transfer surface height for clearing the seat cushion.21
Therefore, the U.S. Access Board should not infer the seat surface height at the front edge is the same as the seat height measured in Dr. Steinfeld’s study. However, these illustrations used in measuring individuals for manual wheelchairs indicate that the U.S. Access Board cannot use the Steinfeld data to make a direct assessment of the table height needed to accommodate wheelchair users effectively.
Based on figures 5-7 below, a 17-inch rear compressed height measured by Steinfeld could easily correspond with a 20- or 21-inch uncompressed seat surface height at the front edge. This could in turn mean that individual users that Steinfeld measured below 19 inches would be able to transfer comfortably to a table surface for which the highest uncompressed surface is 19 inches.
Note further in figure 5 that the rear seat height is considerably lower than the height of the wheelchair wheels, which typically measure 22-26 inches. Any transfer from the rear of the chair would require the user to transfer up and over the wheelchair wheel, again, well above a minimum low-range height of 19 inches.
In addition, a second study commissioned by the U.S. Access Board and evaluated by the Advisory Committee (the Pittsburgh study22) determined that manual wheelchair-users, who are generally the users most likely to have the lowest seated heights, are generally able to accommodate a 2-inch difference in height between one’s wheelchair and a transfer surface. Consequently, even if one posited that the Steinfeld study finding of 17 inches at the rear of the seat compressed corresponded to an uncompressed height of 17 inches at the front edge of the wheelchair, the maximum height of 19 inches at the highest point of a table or chair transfer surface would still fall within the 2-inch differential identified by the Pittsburgh study as accessible to most manual wheelchair users.
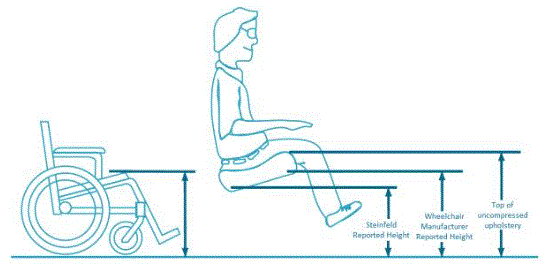
Figure 5: Adapted diagram from the “Paralyzed Veterans of America’s Guide to Wheelchair Selection” showing difference in measured height between Steinfeld report, wheelchair manufacturer’s height per ISO 7176-7, and the uncompressed upholstery measurement height.
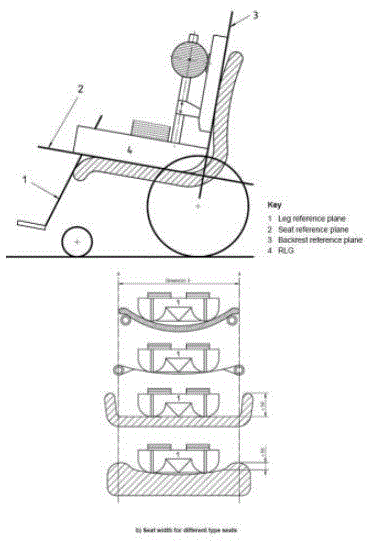
Figure 6: Wheelchair manufacturer’s height and seat construction types (showing variation in compression when person’s weight is applied) per ISO 7176-7
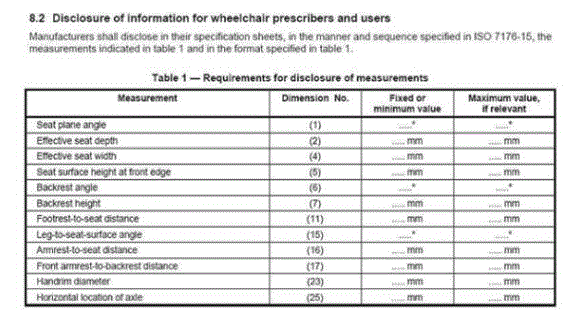
Figure 7: Wheelchair manufacture standardized measurements per ISO 7176-7
Chairs with/without Footrests:
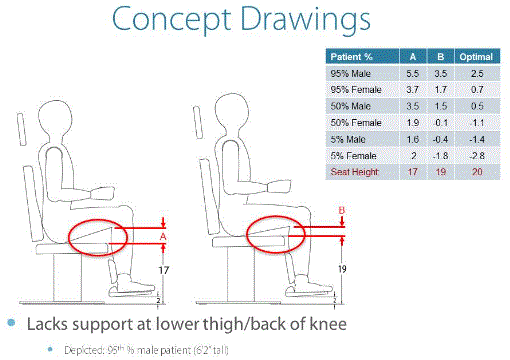
With a 17” transfer height, chairs with footrests, which the patient rests their feet on, such as those used in Otolaryngology, Ophthalmology, Plastic Surgery and others would not be comfortable to sit in for a large number of patients. Domestic UL and international IEC standards dictate a 2” clearance from the floor to the bottom of the footrest. And, with a minimal 1” thick footrest plate, the distance from the top of footrest to the top of the seat would be just 14”. Anthropometric data suggest the heel to popliteal dimension, without shoes, of males is 17.5” and that of females is 15.9” in the 50th percentile. Contact with the back of the thigh and/or the rear of the knee with the top front portion of the seat is vital for patient comfort and is especially important for maintenance of knee and thigh position of a disabled person who may lack control of his/her legs.
Chairs with no footrest plate and only a breaking knee legrest have similar problems with a short legrest portion also. Patient comfort in the entry/exit position can be compromised even after the chair is raised and the back lowered because of the length of the chair top in the supine position. Even with the addition of a flip-up or slide out footrest/legrest extension, the total length of the average knee break chair is estimated to be 60“, and only be able to be a maximum of approximately 68” with extension. We estimate the addition of a manual extension of the footrest could add as much as $750 (15%). An electrical or automatic extension could be a $1300 (26%) addition to the net cost of either a chair with a footrest plate or to one without the plate, which only extends the legrest or calf portion. These chairs would have to have an electrical interlock installed in them to protect the chair and patient from the chair being lowered with the legrest extended. These kinds of additional adjustments and added protection controls tend to discourage the operator from using them and thus the patients could instead be forced to endure a medical procedure in uncomfortable positions.
Adoption of a 19 Inch Height Minimizes Costs to Health Care Providers
In the absence of clear data commending a departure from the existing broadly accepted transfer surface height maximum of 19 inches, information related to costs is especially critical to take into account in determining the minimum standard for medical examination tables and chairs. We estimated the costs of various examination table heights under consideration by the Medical Diagnostic Equipment Technical Advisory Committee using third-party data that represents approximately 80 percent of all distributed examination tables sold in the United States.23
To determine the total number of examination rooms in the United States, we reviewed the CDC National Ambulatory Care Survey, and found that there are approximately 639,000 exam rooms in the United States today.
From this total number, and based on historic trends, we estimate that physicians will build new, or remodel existing, exam rooms at a rate of 4 percent each year, but the total number of examination rooms will remain unchanged from year to year.
Also based on historic trends, we estimate that physicians will replace equipment in 7 percent of their examination rooms each year, including new and replacement medical tables. We also estimate that the average annual inflation rate will be 3% over the next ten years.
Benefits and Costs: Overview
Based on our analysis, we determined that transfer surface height requirements lower than 19 inches would increase the cost of designing and manufacturing examination tables, reduce the rate of adoption of accessible equipment, and increase the health provider’s cost of purchasing accessible equipment.
While we estimate that adopting a lower-range requirement of 19 inches, health care providers will experience a 24 percent price increase. This means that a $5,000 adjustable-height examination table purchased today would cost approximately $6,200 after the Access Board adopts the requirements for knee-crutches and transfer supports because health care providers will need to retrofit existing tables and chairs with the required equipment. The price of that same table would be 39 percent higher than the cost of today’s examination tables if the height standard were lower than 19 inches.
Cost of Equipment
Costs to Lower Minimum Table Height
To be useful for its intended purpose, examination tables and chairs must maintain a high height of at least 32 inches, so that the health care provider has clinical access to the patient. In fact, 37 inches is the standard high height today for OB/GYN examinations. The need to reach heights above 32 inches interferes with efforts to lower the transfer surface height. The mechanical system necessary to raise the table are located in the base of the table, beneath the seating surface. As the difference between the required low-height and the necessary high-height for health care providers’ increases, the mechanics necessary to achieve that adjustable range become more complex and expensive.
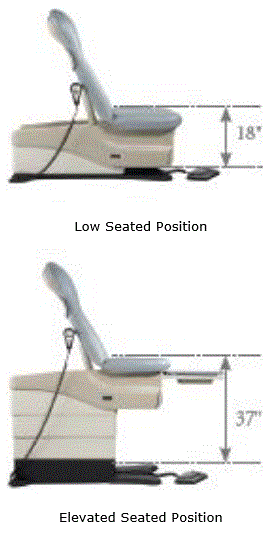
Figure 8: Range of medical table heights
In addition, we took the costs of other requirements that the Access Board is likely to adopt into account. Based on currently marketed products, we estimate the following costs:
-
Transfer Supports (TS): $500 - $1,000 (M301.3.1)
-
Leg Supports (LS): $700 - $1,100 (M301.3.2)
Therefore, the average “upgrade” cost to add transfer and leg supports would be approximately $1,650.
Scoping Scenarios: Range of Possibilities
We do not know what mandates may be put in place in the future to require Access Board standards. However, the U.S. Access Board’s decisions regarding technical requirements for equipment will significantly affect the costs on health care providers and examination table and chair manufacturers.
To illustrate this effect, we considered a broad range of scenarios. For example, if a national mandate were to require one accessible table per physician work area of five exam rooms (a typical physician practice set-up today), that would require a 20 percent adoption rate. We also considered a 100 percent adoption rate to show the full range of potential costs.
If the market adoption rate of adjustable tables were to match a requirement that one table of every five tables meet a 19 inch lower adjustable range when facility construction occurs, there would be a drop in patient access to compliant examination tables of 17% and a savings of $420 million over ten years. However, if the market adoption rate were to match a requirement that ALL new tables meet a 19-inch lower adjustable range when new construction or remodeling occurs, then accessibility would increase by 17% at a cost to health care providers of $1.38 billion over a ten-year period.
By contrast, if the market adoption rate matched a requirement to make one table of every five tables meet an adjustable range lower than 19 inches, there would be a reduction in availability of accessible tables by 35% for a savings of $530 million over ten years. However, if the requirement were to make ALL new tables meet an adjustable range lower than 19 inches when new construction or remodeling occurs, then accessibility would decrease by 1% at a cost to health care providers of $1.52 billion over a ten-year period.
The table and chart below illustrate these examples.
Table 2: Costs of Scoping Scenarios
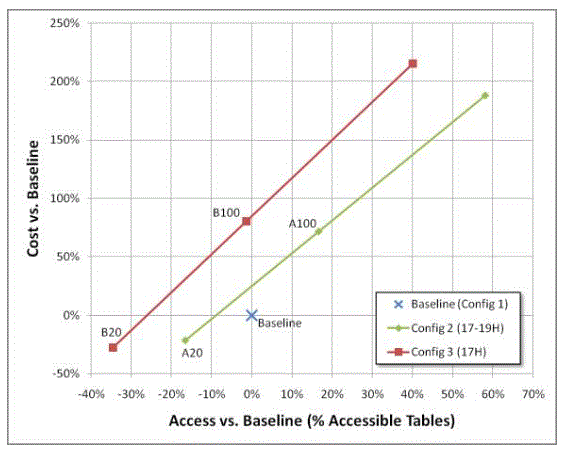
Figure 9: Summary of cost and implementation scenarios of Table 2
Therefore, while establishing these requirements will result in significant costs to health care providers, establishing a lower adjustable height requirement of 19 inches will maximize the percentage of accessible tables and will cost less than requiring an adjustable height lower than 19 inches.
Notes
5. Medical table install base derived from U.S. medical distribution sales data, as provided by Global Healthcare Exchange (GHX), found at http://www.ghx.com/product-pages/solutions/supplier-solutions/sales-data-analytics.aspx
6. See M301.2.1 and M302.2.1.
7. See Architectural and Transportation Barriers Compliance Board. Notice of Proposed Rulemaking: Proposed Accessibility Standards for Medical Diagnostic Equipment. February 8, 2012.
8. Ibid.
9. Ibid.
10. Ibid, citing ANSI/AAMI HE 75, section 16.4.4. ANSI/AAMI HE75 recommends that the height of patient support surfaces "should be easy to adjust (ideally, powered) to suit the needs of health care professionals and patients." ANSI/AAMI HE75 further recommends that the height of patient support surfaces "should be adjustable to a position high enough to accommodate tall health care providers and the range of medical procedures that could occur . . . [and] to a position low enough [19 inches maximum] to allow for the comfort of providers who choose to work in a seated position, to enable patients to keep their feet on the floor while seated, and to accommodate patients who need to transfer laterally between the platform and a chair or wheelchair alongside."
11. See Analysis of Seat Heights for Wheeled Mobility Devices at: http://udeworld.com/analysis-of-seat-height-for-wheeled-mobility-devices. The seat heights ranged from 16.3 inches to 23.9 inches for manual wheelchair users; 16.2 inches to 28.9 inches for power wheelchair users; and 18.8 inches to 25.3 inches for scooter users. Seat heights for males were typically higher than for females. Thirty (30) percent of male manual wheelchair users and 6 percent of male power wheelchair users had seat heights equal to or less than 19 inches. All the male manual wheelchair users and 92 percent of the male power wheelchair users had seat heights equal to or less than 25 inches. Thus, transfer surfaces that are adjustable from 17 inches minimum to 25 inches maximum during patient transfer accommodate significantly more patients who use mobility devices.
12. See Accessible and Usable Buildings and Facilities, ICC/ANSI A117.1-2009.
13. Ibid.
14. Ibid.
15. Ibid.
16. Ibid.
17. Medical table install base derived from U.S. medical distribution sales data, as provided by Global Healthcare Exchange (GHX).
18. Some tables may require installation of a modified top to meet the 19” standard but would not require changing out the installed base.
19. See Analysis of Seat Heights for Wheeled Mobility Devices at: http://udeworld.com/analysis-of-seat-height-for-wheeled-mobility-devices. The seat heights ranged from 16.3 inches to 23.9 inches for manual wheelchair users; 16.2 inches to 28.9 inches for power wheelchair users; and 18.8 inches to 25.3 inches for scooter users. Seat heights for males were typically higher than for females. Thirty (30) percent of male manual wheelchair users and 6 percent of male power wheelchair users had seat heights equal to or less than 19 inches. All the male manual wheelchair users and 92 percent of the male power wheelchair users had seat heights equal to or less than 25 inches. Thus, transfer surfaces that are adjustable from 17 inches minimum to 25 inches maximum during patient transfer accommodate significantly more patients who use mobility devices.
20. Available at http://www.wheelchairnet.org/WCN_ProdServ/Docs/PDF/AXbook_Sec4a.pdf
21. Ibid.
22. Human Engineering Research Laboratories, University of Pittsburgh, The Impact of Transfer Set-Up on the Performance of Independent Transfers: Final Report. Available at: http://herl.pitt.edu/ab/transfer_assessment_report.pdf (visited May 22, 2013).
23. Medical table install base derived from U.S. medical distribution sales data, as provided by Global Healthcare Exchange (GHX), found at http://www.ghx.com/product-pages/solutions/supplier-solutions/sales-data-analytics.aspx
How to Measure Transfer Surface Height
Regardless of the final decision on the low height measurement, the Subcommittee agreed that the height of the transfer surface would be measured in its uncompressed state.
Many medical examination rooms are equipped with fixed height examination tables, with a typical 32-inch seat height. However, these fixed heights tables do not allow for independent transfer of patients who use wheeled mobility devices (WMD). To solve this problem, manufacturers have designed adjustable height examination tables to work with a variety of WMD’s. Manufacturers designed the shape of the seat for these tables to meet both patient accessibility and clinical needs, resulting in a complex, contoured shape.
Because of these complex shapes, it is necessary to create a standard method by which to measure table seat dimensions. These proposed measurement techniques would apply equally to tables (M301) and chairs (M302).
Several necessary features determine the shape of an examination table seat:
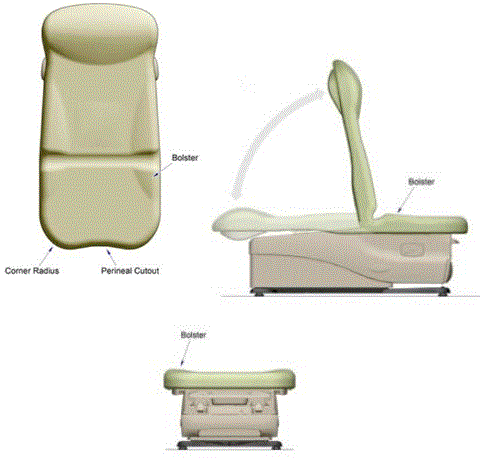
The perineal cut-out provides access to the perineum for gynecological and urological examinations.
The corner radii allow for closer wheelchair positioning to facilitate independent transfer by minimizing gaps. The corner radii also eliminate seams in the upholstery, which improves longevity, but more importantly also improves asepsis and infection control.
Bolsters improve patient comfort and stability when seated on table. Note that the design minimizes the bolsters at the front half of the seat in order to promote ease of transfer.
Note that these features are widely used in both tables and chairs. Beds, stretchers, and other types of equipment will have unique features that determine the shapes of their patient support surfaces.
Figure 10: Features of the countered shape of a medical examination table
The design of the corner radii allows closest possible position for wheelchair transfers, minimizing potential gaps and improving the patient’s ability to transfer independently.
In the diagram below, the upper wheelchair illustrates a typical side transfer, which may optionally utilize a transfer board. The lower wheelchair illustrates a typical diagonal transfer.24
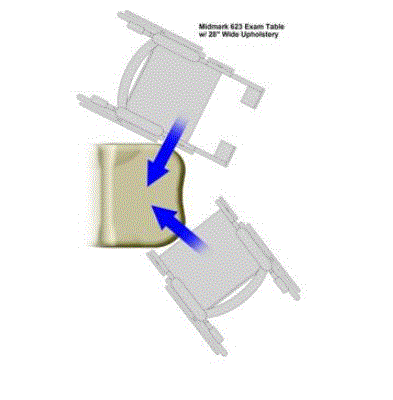
Figure 11: Corner radii of a medical examination table
The depth and width are measured along the centerlines of the seat, and the height from the floor to the highest point of the transfer surface:
-
The depth is measured between the perineal cutout and the hinge point at the back of the seat.
-
The width measured across the seat at the midpoint between the seat hinge and the front of the seat.
-
The height is measured at the highest point of the seat, inclusive of bolsters, with the foam in an uncompressed state. Note that this measurement would be at the highest point on the seating surface, which may not necessarily be at the centerline of the seat.
Notes
24. Examples of such transfers can be viewed here: http://www.youtube.com/watch?v=qivOb_V6IgA
Transfer Supports Location and Configuration M301.3 and M302.3
Location of the transfer supports shall be a minimum of 6 inches to a maximum of 19 inches from the top of the transfer surface and ensure that any entrapment issues are addressed as per 606.1.252. The length of the transfer support shall be a minimum of 15 inches long and positioned so that the transfer support overlaps the length of the transfer surface by 80% at a maximum distance from transfer surface of 1 ½ inches. The shape (contours and curves along length for ergonomics, not cross sectional profile) was kept open to give manufacture flexibility in the design as the direction of positioning (i.e. horizontal, angled, etc.). Transfer supports shall be mounted on both sides of the transfer surface and must be movable or removable so they can be out of way of transfer. The rating is for 250 pounds of force in direction of use as per IEC 60601 & BIFMA.
We recommended height adjustability of the transfer support but did not mandate it to give manufactures design options.
Gripping Surface Cross Section and Clearances M301, M302, and M305.2
Gripping surface for transfer supports shall be free of sharp or abrasive elements and shall have rounded edges per ADA and ABA 2004 Guidelines Section 609.5. Interruptions along the bottom of the transfer support gripping surface will not obstruct more than 20% of its length per ADA and ABA 2004 Guidelines Section 505.6. The transfer supports shall be located at a maximum distance from transfer surface of 1 ½ inches.
Armrests M302.3.2
Armrests are recommended but not required. If provided armrests cannot interfere with transfer supports during the transfer. Dual transfer support/armrest mechanisms are allowed as long as the transfer support meets all of the requirements for transfer supports.
Notes
1. The Impact of Transfer Setup on the Performance of Independent Transfers: Final Report. Presentation to US Access Board. Washington, DC. 2011
2. D’Souza, Clive and Edward Steinfeld, IDeA Center. Analysis of Seat Height for Wheeled Mobility Devices. 2011.
3. The Impact of Transfer Setup on the Performance of Independent Transfers: Final Report. Presentation to US Access Board. Washington, DC. 2011
4. Jensen, M.P., Molton, I.R., Groah, S.L., Campbell, M.L., Charlifue, S., Chiodo, A., Forchheimer, M., Krause, J.S., & Tate, D. (2011). Secondary Health Conditions in Individuals Aging with SCI: Terminology, Concepts, and Analytic Approaches. Spinal Cord, 50(5): 373-378.
Groah, S.L., Charlifue, S., Tate, D., Jensen, M.P., Molton, I.R., Forchheimer, M., Krause, J.S., Lammertse, D.P., & Campbell, M. (2012). Spinal Cord Injury and Aging: Challenges and Recommendations for Future Research. American Journal of Physical Medicine & Rehabilitation, 91(1): 80. doi: 10.1097/PHM.0b013e31821f70bc. Available from: http://journals.lww.com/ajpmr/Abstract/2012/01000/Spinal_Cord_Injury_and_Aging__Challenges_and.10.aspx. Accessed December 18, 2012.
Turk M. Secondary conditions and disability. In: Field MJ, Jette AM, Martin L (eds). Workshop on disability in America. A new look. Summary and background papers. Board on Health Sciences Policy, Institute of Medicine of the National Academies, The National Academies Press: Washington DC, 2006, pp. 185–193.
Kemp, B.J., & Mosqueda, L. (Eds.) (2004). Aging with a Disability: What the Clinician Needs to Know. Baltimore, MD: Johns Hopkins University Press.
Kailes, J. (2000). Health, Wellness and Aging with Disability, KAILES - Publications, http://www.jik.com/resource.html, jik@pacbell.net This email address is being protected from spambots. You need JavaScript enabled to view it. .
Kailes, J. (1995). "Midlife Cripdom: Getting Fewer Miles per Gallon?" The Disability Rag 16(4).
Kailes, J. (2001). Aging with Disability - Good News and Bad News. Western U-View. XX: 17.
5. Medical table install base derived from U.S. medical distribution sales data, as provided by Global Healthcare Exchange (GHX), found at http://www.ghx.com/product-pages/solutions/supplier-solutions/sales-data-analytics.aspx
6. See M301.2.1 and M302.2.1.
7. See Architectural and Transportation Barriers Compliance Board. Notice of Proposed Rulemaking: Proposed Accessibility Standards for Medical Diagnostic Equipment. February 8, 2012.
8. Ibid.
9. Ibid.
10.Ibid, citing ANSI/AAMI HE 75, section 16.4.4. ANSI/AAMI HE75 recommends that the height of patient support surfaces "should be easy to adjust (ideally, powered) to suit the needs of health care professionals and patients." ANSI/AAMI HE75 further recommends that the height of patient support surfaces "should be adjustable to a position high enough to accommodate tall health care providers and the range of medical procedures that could occur . . . [and] to a position low enough [19 inches maximum] to allow for the comfort of providers who choose to work in a seated position, to enable patients to keep their feet on the floor while seated, and to accommodate patients who need to transfer laterally between the platform and a chair or wheelchair alongside."
11.See Analysis of Seat Heights for Wheeled Mobility Devices at: http://udeworld.com/analysis-of-seat-height-for-wheeled-mobility-devices. The seat heights ranged from 16.3 inches to 23.9 inches for manual wheelchair users; 16.2 inches to 28.9 inches for power wheelchair users; and 18.8 inches to 25.3 inches for scooter users. Seat heights for males were typically higher than for females. Thirty (30) percent of male manual wheelchair users and 6 percent of male power wheelchair users had seat heights equal to or less than 19 inches. All the male manual wheelchair users and 92 percent of the male power wheelchair users had seat heights equal to or less than 25 inches. Thus, transfer surfaces that are adjustable from 17 inches minimum to 25 inches maximum during patient transfer accommodate significantly more patients who use mobility devices.
12.See Accessible and Usable Buildings and Facilities, ICC/ANSI A117.1-2009.
13. Ibid.
14. Ibid.
15. Ibid.
16. Ibid.
17. Medical table install base derived from U.S. medical distribution sales data, as provided by Global Healthcare Exchange (GHX).
18. Some tables may require installation of a modified top to meet the 19” standard but would not require changing out the installed base.
19. See Analysis of Seat Heights for Wheeled Mobility Devices at: http://udeworld.com/analysis-of-seat-height-for-wheeled-mobility-devices. The seat heights ranged from 16.3 inches to 23.9 inches for manual wheelchair users; 16.2 inches to 28.9 inches for power wheelchair users; and 18.8 inches to 25.3 inches for scooter users. Seat heights for males were typically higher than for females. Thirty (30) percent of male manual wheelchair users and 6 percent of male power wheelchair users had seat heights equal to or less than 19 inches. All the male manual wheelchair users and 92 percent of the male power wheelchair users had seat heights equal to or less than 25 inches. Thus, transfer surfaces that are adjustable from 17 inches minimum to 25 inches maximum during patient transfer accommodate significantly more patients who use mobility devices.
20. Available at http://www.wheelchairnet.org/WCN_ProdServ/Docs/PDF/AXbook_Sec4a.pdf.
21. Ibid.
22. Human Engineering Research Laboratories, University of Pittsburgh, The Impact of Transfer Set-Up on the Performance of Independent Transfers: Final Report. Available at: http://herl.pitt.edu/ab/transfer_assessment_report.pdf (visited May 22, 2013).
23. Medical table install base derived from U.S. medical distribution sales data, as provided by Global Healthcare Exchange (GHX), found at http://www.ghx.com/product-pages/solutions/supplier-solutions/sales-data-analytics.aspx
24. Examples of such transfers can be viewed here: http://www.youtube.com/watch?v=qivOb_V6IgA
Accessibility Standards for Medical Diagnostic Equipment
Medical Diagnostic Equipment Accessibility Standards
Sub-Committee Recommendations - Imaging Equipment
Final Report
June 2, 2013
The Subcommittee’s technical recommendations for imaging equipment (except Mammographic equipment) have taken into consideration the following technical criteria proposed in Chapter 3, part 1195 to Title 36 of the Code of Federal Regulations. Especially, Section M301, Diagnostic Equipment Used by Patients in Supine, Prone, or Side-Lying Position. However, given the enormous diversity of imaging equipment needed to achieve the broad range of diagnostic objectives Sections M302, Diagnostic Equipment used by Patients in Seated Position; M303, Diagnostic Equipment used by Patients Seated in a Wheelchair; and M304, Diagnostic Equipment used by Patients in Standing Position were considered and are noted in this report.
4. Perspectives of Equipment Types
4.3 Diagnostic Imaging Equipment
The diagnostic imaging equipment covered by this subcommittee had an extraordinary breadth and depth of its diversity of design, configurations, and principles of operation. This is the direct result of enormous variety of diagnostic tasks, clinical indications, and patient populations this equipment has been designed to serve both in a general manner as well as configurations that are highly optimized to provide optimized results for a particular clinical need.
The types of equipment covered and evaluated by the imaging subcommittee include:
-
Computed Tomography (CT)
-
Magnetic Resonance (MR)
-
Nuclear Medicine (Scintigraphy & Single Photon Emission Computed Tomography) (NM)
-
Positron Emission Tomography (PET)
-
X-Ray Fluoroscopy
-
X-Ray Radiography
-
X-Ray Interventional
-
X-Ray Mobiles
-
X-Ray C-arms
-
Dual-energy X-ray Absorptiometry (DXA)
-
X-Ray Mammography Biopsy Tables
-
PET/CT Combined Systems
-
NM/CT Combined Systems
-
PET/MR Combined System
These represent all virtually all diagnostic imaging systems except conventional Mammographic systems which were addressed by their own subcommittee and Ultrasound systems that due to their portability do not fall under the scope of these proposed standards.
This equipment consist mostly of large capital equipment, uses ionizing radiation (or a very strong magnetic field) to produce the images, has many years of service life, and represents significant investment to the facility. This equipment is also primarily permanently mounted in a fixed installation that special room siting design needs to be performed for. This special siting is the result of a variety of factors that include shielding of ionizing radiation or magnetic fields and specialized high power capacity electrical service. These systems do not have patient “operable parts” (i.e. the patient does not activate, deactivate, or adjust the equipment).
This equipment are all prescription use only devices, meaning that one must have a physician’s order before a person may receive an exam. The devices all must be operated by a trained and qualified technologist, who must be present during the exam to aid all patients onto the table, explain the exam process, and aid in properly positioning the patient.
This equipment represents US Food and Drug Administration (FDA) Class II medical devices that need pre-market notification to FDA (510(k) clearance) prior to being placed on the market. They must be design and manufactured under the Quality System Regulations for medical devices, 21CFR820, that includes design controls and good manufacturing practices. They must be tested and certified by an OSHA credentialed Nationally Recognized Testing Laboratory to demonstrate that they meet the basic safety and essential performance required by IEC60601-1 as well as the applicable IEC 60601-1 series of collateral and particular standards. The devices that produce X-rays must also be certified to FDA to meet the applicable performance standards for radiation safety found in 21CFRSubchapter J. The design process must conform include risk-management in accordance with ISO 14971. Radioactive Sources and Radiopharmaceuticals used for PET and NM are also regulated by the Nuclear Regulatory Commission.
A diagnostic imaging device’s the transfer surface (table) is both imaged through and also positions the patient during the imaging process, hence it plays in integral role in the diagnostic exam and is critical to achieving accurate diagnostic results and controlling radiation exposure to the patient. This, along with the mechanical, electrical, and physics aspects and needs of diagnostic imaging equipment create for a wide variety of designs and some inherent limitations to table (transfer surface) design possibilities.
The following is a rough grouping of diagnostic imaging devices that was used to help evaluate the criteria:
Equipment with bores: CT, PET, PET/CT, NM, NM/CT - Here the table plays an integral part in achieving the sub-mm dynamic positioning accuracy needed during the scan.
MR - This shares same aspects as equipment with bores, but has special considerations due to the very strong magnetic field.
DXA - This equipment necessitates positioning the x-ray source under the patient in a fixed, known geometry for diagnostic effectiveness and radiation dose concerns.
Conventional XR and Fluoroscopy - This equipment has rectangular, radio-translucent tables that may translate in both directions in the horizontal plane.
Mobile XR - These systems can be moved to the patient and can utilize detachable detectors that often can be placed behind the patient anatomy to be imaged without significant patient movement.
Interventional XR - This type of equipment, such as that used in cath labs and C-arms, has virtually all patients under some form of sedation prior to transfer, and is used in an invasive “interventional-like” procedure after initial diagnostic findings.
Prone breast biopsy tables - This unique design needs to accommodate room for the physician underneath the patient, patients may have some form of sedation, and is used in an invasive “interventional-like” procedure after initial diagnostic findings.
Diagnostic imaging tables mostly fall into two main groupings. One group is those tables used with “equipment with bores”, such as CT, MR, and NM systems. These tables tend to be long and relatively narrow in order to move the patient into and fit through the bore. They are typically rated for patients in excess of 400 lbs. They are capable of adjusting with the high precision (sub millimeter) accuracy needed for accurate diagnostic information both vertically for both patient loading and unloading procedures, and horizontally in one direction (into the bore).
The other group is those tables used on X-Ray system. These too are typically rated for patients in excess of 400 lbs, but are wider than those used with equipment with bores and in many cases are able to move horizontally in two directions. The may not be designed to adjust vertically, but some are designed to rotate to place the patient in a more vertical position needed for specific diagnostic exam needs. Tables for DXA equipment present a noteworthy uniqueness because for both diagnostic and mechanical reasons, they are fixed and do not adjust in any direction.
It must be noted that for patient support devices must meet applicable safety factors as delineated in IEC 60601-1. These factors typically range from 4x to 8x. This means a patient table labeled to support a 500 lb patient must actually be designed and tested at up to 4000 lbs. This has significant implications for adjustable height table design as many design loose mechanical advantage as they go lower.
Many X-Ray systems have imaging components such as X-Ray tubes, high voltage generators, and/or detectors located under the table (transfer surface). The fact that the tables used for diagnostic imaging equipment must not only support large weights, precisely position and image, and potentially accommodate imaging components make redesign of imaging tables challenging at best, but perhaps in some cases infeasible. In all cases, due to the complexity of the equipment and the regulatory requirements, a redesign of an imaging system or its table would require a multi-year process.
One type of equipment stood out as unique are systems used for interventional and biopsy procedures. Both interventional procedures and biopsies are technically considered “diagnostic” because they can provide diagnostic information. These systems however, frequently require all patients to under some form of sedation prior to transfer. Because of the use of sedation, their invasiveness, and that these exams are a secondary follow-up to a primary diagnostic finding, they seem more related treatment and hence the subcommittee and the full advisory committee believes they should be considered out of scope for these standards and hence exempted.
For those systems that will be subject to the new standards, there are certain constraints and performance considerations:
-
Must maintain same degree of diagnostic performance for all patients.
-
Huge variation of clinical applications and patient needs.
-
Technical and diagnostic constraints.
-
Must maintain accessibility for all patients and patient conditions.
-
Must maintain health care professional access to patient and patient support equipment.
-
Must maintain infection control constraints.
-
Must continue to adhere to FDA and international standards.
-
There is not a one-size-fits-all solution.
All current diagnostic imaging equipment does not meet a minimum transfer height of 17 inches, however some equipment with bore tables do currently meet 19 inches. However, with equipment redesign there are some that may (e.g. CT), but most will encounter a significant technical or diagnostic barrier if the actual transfer surface (table) must be altered. Creative and alternative solutions are needed to increase independent transfer for maximum adoption by facilities (e.g. “accessibility packages”).
The subcommittee believes alternate criteria or “accessibility packages”, to strive for equivalent facilitation, will be needed to best improve independent access in the most meaningful way while adhering to the above constraints and considerations. Accessibility Packages would include accessory components, ancillary equipment, and/or siting design requirements. Accessibility packages may be able a timely, cost effective solution that may also be able to be applied to existing equipment to increase accessibility.
Section 201(h) of the Federal Food Drug & Cosmetic Act includes accessories with the definition of a medical device, and the IEC 60601-1 international standard for medical electrical equipment also identifies that medical electrical equipment includes those accessories that are necessary to enable the normal use of the equipment which includes facilitation of its use.
Section 5 of this report contains some figures of conceptual ideas for accessibility accessories.
Patient Positions during Diagnostic Imaging
The vast majority of diagnostic imaging exams are conducted while the patient is lying on the table; hence the focus of the subcommittee was for the proposed standards in Section M301. However, given the enormous diversity of imaging equipment needed to achieve the broad range of diagnostic objectives Sections M302, Diagnostic Equipment used by Patients in Seated Position; M303, Diagnostic Equipment used by Patients Seated in a Wheelchair; and M304, Diagnostic Equipment used by Patients in Standing Position were considered and are noted in this report.
There are some Nuclear Medicine systems that have a unique design for convenience where the system’s table can pivot out of the way to allow a scan while a patient seated (in a chair or wheelchair). However, given the clinical input from the radiologist that presented to the advisory committee, an equivalent diagnostic exam, in all cases may be obtained while a patient is on the table. As such both the subcommittee and the full advisory committee agreed that for these types of Nuclear Medicine systems, only the M301 criteria should be applied.
Some specialized MR and possibly other modality devices designed and used for extremity scans may not be provided with a table. In such cases the patient chair should comply with the conclusions of the tables and chairs subcommittee to the extent practical while still meeting the diagnostic needs.
Some X-Rays exams are performed with a wall stand in use where the patient is asked to stand. In these situations M304 would apply. However it is likely that the standing supports may need to be an accessory or a mounting in the room. Additionally, given that these supports may also need to serve the diagnostic purpose of a positioning aid some of the dimensions proposed in M305.3 may need to be adjusted in order to maintain diagnostic efficacy.
5. Recommendations
5.3 Diagnostic Imaging Equipment
One type of equipment stood out as unique are systems used for interventional X-Ray and breast prone biopsy procedures. Both interventional procedures and biopsies are technically considered “diagnostic” because they can provide diagnostic information. These systems however, frequently require all patients to under some form of sedation prior to transfer. Because of the use of sedation, their invasiveness, and that these exams are a secondary follow-up to a primary diagnostic finding, they seem more related treatment and hence the subcommittee and the full advisory committee believes they should be considered out of scope for these standards and hence exempted.
As discussed in Section 4.3, the requirements for diagnostic imaging equipment do not apply to patients in a seated (chair or wheelchair) when the imaging system is provided with and/or intended to be used for patients in prone, supine, or Side-Lying Position. Some X-Rays exams are performed with a wall stand in use where the patient is asked to stand. In these situations M304 would apply. However it is likely that the standing supports may need to be an accessory or a mounting in the room. Additionally, given that these supports may also need to serve the diagnostic purpose of a positioning aid some of the dimensions proposed in M305.3 may need to be adjusted in order to maintain diagnostic efficacy.
M301.2.1 Transfer Surface Height
Subcommittee Recommendation:
The imaging subcommittee decided to not specifically come up with a recommendation for transfer height, rather leaving that to the full committee’s determination and then have it applied to diagnostic imaging equipment.
Rationale: For many types of imaging equipment it will not be feasible to provide a transfer surface meeting the considered minimum height whether it is 17, 18, or 19 inches. The same is true for height adjustability for DXA and some X-Ray systems. In those instances an alternate means of access will need to be provided such as the use of auxiliary/ancillary equipment and accessories (“Accessibility Package”).
Some CT tables can currently meet an 18 or 19 inch height and with redesign, more tables for use with equipment with bores would be able to. Most tables for use with equipment for bores are height adjustable. The limitations with these types of tables are that they are typically designed to accommodate >400 lb patients while performing sub-millimeter diagnostic positioning. These tables need to typically be designed with an 8x safety factor and hence have sizable support mechanisms, some of which are designed such that as the table is lowered, mechanical advantage is lost. Every inch is significant from a design perspective.
Tables used for MR imaging must also meet the requirements of being in very strong magnetic fields. Additionally these magnetic fields also preclude use of a patient’s mobility device in the exam room. However, currently there are MR table designs that are detachable and can be moved outside of the MR room where the patient can transfer. In such cases these tables become very similar to CT tables for accessibility considerations.
Many X-Ray systems have imaging components such as X-Ray tubes, high voltage generators, and/or detectors located under the table (transfer surface). X-Ray tables are also typically rated for patients in excess of 400 lbs, but are wider than those used with equipment with bores and in many cases are able to move horizontally in two directions. The may not be designed to adjust vertically, but some are designed to rotate to place the patient in a more vertical position needed for specific diagnostic exam needs. Tables for DXA equipment present a noteworthy uniqueness because for both diagnostic and mechanical reasons, they are fixed and do not adjust in any direction.
Section 4.3 of this report contains additional considerations from the imaging subcommittee.

Figure 5.3-1: This is picture of a CT system (this one has decals on it for use in a children’s hospital). It is also representative of a MR table. The table on this particular model is 7+ ft long, about 24 inches wide, and has a minimum height of about 18 inches. Note the emergency extraction handle at the foot end of the table. Also note that there is not structural material under the table side covers where transfer supports could sufficiently be anchored.

Figure 5.3-2: This is a picture of a PET/CT system. The PET gantry is located behind the CT gantry, under a single cover. The patient table is virtually identical to the CT table in Figure 5.3-1, however it must be mounted on a transporter (adding 4-5 inches it the minimum height) in order to move it closer to the gantry for the PET scan. Simply having a longer cradle is not done because the cradle must be of material that is virtually transparent to X-rays, and this requirement results in there being some table “sag” when it is extended with a patient on it. A longer cradle will have more sag to the point of not being diagnostically acceptable…hence the transporter.
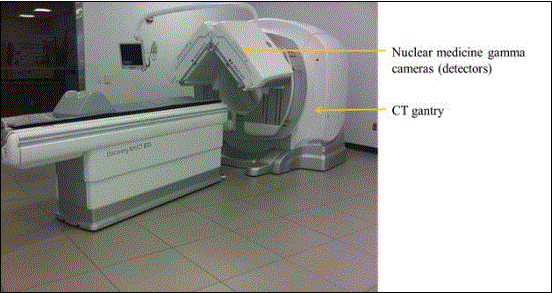
Figure 5.3-3: This is a picture of a NM/CT system. The NM detector heads are located in front of the CT scanner. These heads are able to rotate 360 degrees. The patient table top design is similar to that of a CT system; it is about 24 inches wide in total. On this model the minimum height is 23.2 inches. This is due to the different type of lifting mechanism employed because the table base just needs to move straight up and down. This type of design is also found on other manufactures’ CT and MR tables. The table side covers have the same issues discussed for the CT table in Figure 5.3-1.
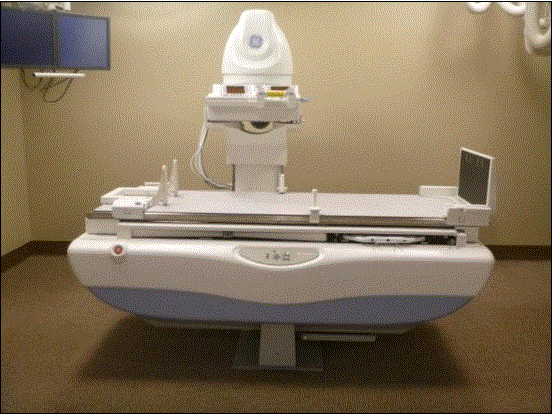
Figure 5.3-4a

Figure 5.3-4c
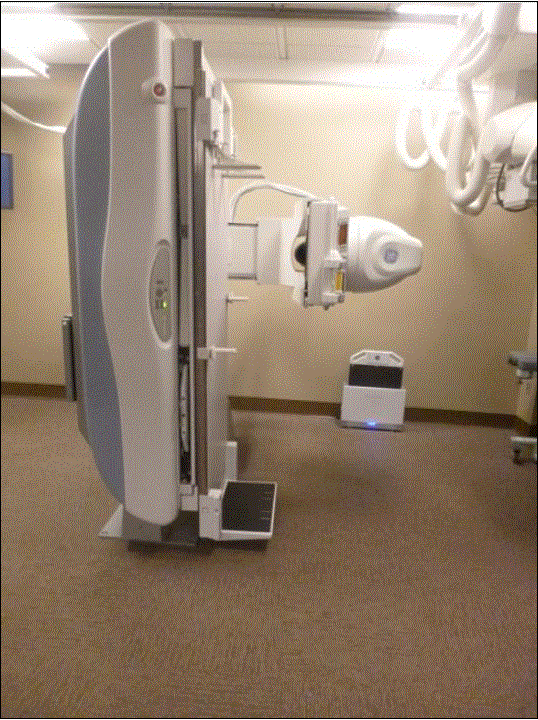
Figure 5.3-4c
Figure 5.3-4: These pictures show an Angulating Radiographic and Fluoroscopic Exam Table whose fixed height is approximately 34.5 inches. The height is the result of the design being able to angulate to perform certain types of diagnostic exams and also to accommodate imaging components under the table such as X-Ray tubes, high voltage generators, and detectors. The table surface is also able to move in two directions horizontally. Also note the equipment imaging components on the opposite side of where the patient transfers.
Figure 5.3-5a
Figure 5.3-5b
Figure 5.3-5: These pictures show a Dual Energy X-ray Absorptiometry (DXA) system for Osteoporosis assessment. The table heights are fixed due to the diagnostic need for a fixed geometry. The table heights are typically 25 - 28 inches and are dictated by the needing the X-Ray source below the table for diagnostic and radiation dose considerations. Also note the equipment imaging components on the opposite side of where the patient transfers.
The subcommittee and full committee believes alternate criteria or “accessibility packages”, to strive for equivalent facilitation, will be needed to best improve independent access in the most meaningful way while adhering to the above constraints and considerations. Accessibility Packages would include accessory components, ancillary equipment, and/or siting design requirements. Accessibility packages may be able a timely, cost effective solution that may also be able to be applied to existing equipment to increase accessibility.
The following figures show some concepts of accessories to address table height that could be included an accessibility package. The accessory or installation would result in decreasing the distance between the transfer surface and the surface where the mobility device is located.
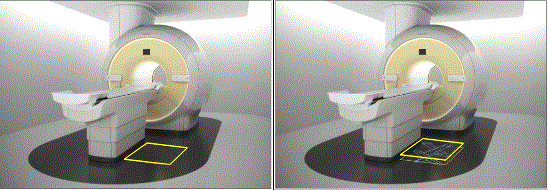
Figure 5.3-6: Flush mounted scissors lift concept (not to scale). The left side shows a flush mounted lift as in the down position while the right side shows it in its elevated position. The lift would need to appropriately sized and ramped and include edge protection.
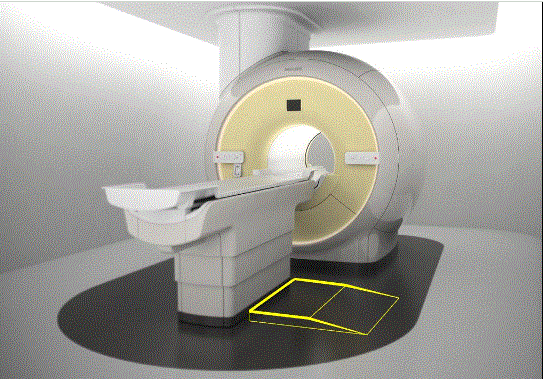
Figure 5.3-7: Elevated platform or possibly “full” floor concept (not to scale). This drawing illustrates the idea of raising the floor instead of lowering the table. This could possibly be accomplished either by a raised platform on the transfer side as shown in the drawing or by building up the floor in the entire room. If an installation is new, it may also be possible to lower the mounting surface of the equipment. An elevated platform would need to appropriately sized and ramped and include edge protection.
M301.2.2 Transfer Surface Size
Subcommittee Recommendation: (Also agreed to by the full committee).
A. The minimum size of the transfer surface is 28 inches wide minimum and 21 inches deep minimum and is located such that the long dimension of 28 inches is be located parallel to the patient scanning/imaging table side at a location designated by the equipment manufacturer.
B. When the transfer surface is located as described above in “A”, the width of the patient scanning/imaging table (side to side) at the designated transfer location should be 28 inches minimum or the maximum possible/practicable, but in all cases a minimum of 21 inches. Note: Alternate means of temporarily widening the table at the designated transfer location during transfer may be feasible.
Rationale: Diagnostic imaging equipment is accessed by all individuals from one of the long sides of the table. Dependent on room layout and other ancillary equipment, both long sides of equipment with a bore tables may be available for transfer. However, many X-Ray system tables and all DXA tables will have part of the imaging equipment support located on one of the long sides and only all transfers are made from the other.
Because diagnostic imaging equipment uses tables for imaging and the tables are always accessed from their long sides, all current equipment meets the 28 inch minimum width and also meets the 21 inch minimum depth for item “A”.
All X-Ray tables meet the 28 inch table width for item “B”. However, because of considerations such as bore size, not all tables used with equipment with bores meet the 28 inch width criteria from “B” (but they do all meet the 21 inch minimum).
The subcommittee created the additional 28 inch table width criteria in “B” because the transfer surface in diagnostic imaging equipment is different than that of exam tables or chairs. With an exam table or chair there is additional table or chair “beyond” the transfer depth. However, with some imaging equipment, due to the transfer from the long side of the table, there may not be any more table beyond the transfer depth.
The subcommittee understands the potential practical limitations that may be encountered on tables used with equipment with bores and therefore only had the 28 inch width criteria for “B” be located at the manufacture designated transfer location.
The possible narrowness of some diagnostic imaging tables was addressed by the subcommittee by their recommendations for M301.3 for transfer supports. The subcommittee sees their recommendations for M301.2.2 transfer surface size and M301.3 to work together to facilitate independent transfers.
Below is a schematic to help illustrate the proposed requirements.
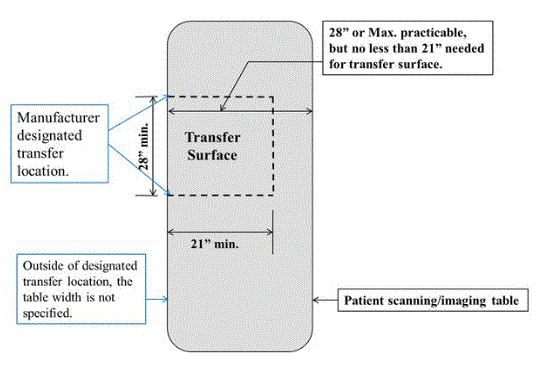
Figure 5.3-8: Schematic of Transfer Surface Size for Diagnostic Imaging Equipment
M301.2.3 Transfer Surface Transfer Sides
Subcommittee Recommendation: (Also agreed to by the full committee.)
The option to transfer will be required only on one long side of the patient scanning/imaging table and not at the “foot” or “head” end of such surfaces.
Note: most installations can accommodate transfer from either long side for equipment with bores (siting and site dependent). However X-ray systems are likely to, and DXA systems will have equipment obstructions on one long side. Therefore, where feasible within the medical equipment design, the ability to have transfer access from two sides (either two long sides or a long and short side) is desired.
Rationale: Diagnostic imaging equipment is accessed by all individuals from one of the long sides of the table. Dependent on room layout and other ancillary equipment, both long sides of equipment with a bore tables may be available for transfer. However, many X-Ray system tables and all DXA tables will have part of the imaging equipment support located on one of the long sides and only all transfers are made from the other.
The head and foot ends of the table are likely to have obstructions such as extraction handles, the gantry, or patient positioning devices. Imaging tables are usually not intended to be access from the head or foot end because the patient would need to “scoot” a long distance to get into the proper position for their exam.
M301.3.1 Transfer Supports
Subcommittee Recommendation: (Also agreed to by the full committee.)
A) For transfer depths less than or equal to 24 inches a transfer support will be located opposite the transfer side.
Note: if transfer is possible from either of the long sides of the table, then a transfer support should be able to be located (or relocated) to the side opposite where the actual transfer is occurring.)
A.1) A transfer support will extend horizontally along the side of the patient scanning/imaging table at least the minimum width of the transfer surface, but in all cases a 28 inches minimum. It will be located at the designated transfer location.
Note: The transfer support will likely need to be separate from the patient imaging table and this is acceptable if such a support meets all relevant technical criteria and is designed to address entrapment hazards. Also note that some equipment has bi-directional table movement which may complicate or in some cases possibly prevent location of a support.
B) For transfer depths greater than 24 inches a positioning support will be located opposite the transfer side.
Note 1: As with other patient positioning aids, a positioning support’s load bearing design may be different than that of a transfer support. These requirements are already addressed by international standards that medical device manufacturers need to follow (e.g. risk management (ISO 14971); IEC60601-1; and use of IEC60601-2-52 as a possible reference.
Note 2: if transfer is possible from either of the long sides of the table, then a positioning support should be able to be located (or relocated) to the side opposite where the actual transfer is occurring.
B.1) A positioning support will extend horizontally along the side of the patient scanning/imaging bed/table and be 12 - 16 inches in length and 3 - 6 inches above the transfer surface. It will be located at a position designated by the manufacturer for optimal positioning assistance.
Note: The positioning support may need to be separate from the patient imaging table and this is acceptable if such a support meets all relevant technical criteria and is designed to address entrapment hazards. Also note that some equipment has bi-directional table movement which may complicate or in some cases possibly prevent location of a support.
C) The maximum distance from the transfer surface to either the transfer support or the positioning support is 1.5 inches. However, an exception of up to 3 inches is acceptable for foldable, collapsible, removable, and articulating supports.
Rationale: The subcommittee created this two part standard because the transfer surface in diagnostic imaging equipment is different than that of exam tables or chairs. With an exam table or chair there is additional table or chair “beyond” the transfer depth. However, with some imaging equipment, due to the transfer from the long side of the table, there may not be any more table beyond the transfer depth. Hence for the cases of narrow (≤ 24”) tables a larger and more substantial transfer support is called for. This transfer support is intended to be used to facilitate transfers and as a prevention for a patient from falling over the table’s opposite side. It there must be designed for this use case. 24 inches was chosen based on input of a person’s maximum reach.
For wider tables (>24”), a transfer support would not be usable because of its distance from the patient using a mobility device. However, the subcommittee saw the need to still have some form of support bar on the opposite side of the table to be used as a positioning aid once the transfer is completed. Because the patient would now be primarily supported by the table, this positioning aid would need to be designed for the loadings of positioning-only use case. The location of this positioning aid will be determined by the manufacture because they have the best knowledge of the particular equipment’s clinical use cases and positioning needs.
The subcommittee recognizes that diagnostic imaging tables either due to bi-directional horizontal movement such as for some X-Ray tables or a lack of sufficient structural integrity on the side of the table may either make it technically very challenging to provide a positioning or transfer support. One means to address this may be by not mounting the support to the table itself, rather the support could be an accessory as part of an accessibility package.
Another critical factor that must be considered is the ability of HCP’s to access the patient during an imaging exam for proper position, administration of imaging agents or other drugs, patient monitoring, etc. Additionally, because in most cases the tables on diagnostic imaging move during the exam and there may be both moveable and stationary parts of the table, design consideration must be given to avoid tubing and other such items that may be attached to the patient from getting caught in a tableside support and pulled out of a patient.
The allowed distance to the transfer or positioning support was chosen to consistent with that of developed by the stretchers subcommittee.
Below is a schematic to help illustrate the proposed requirements.
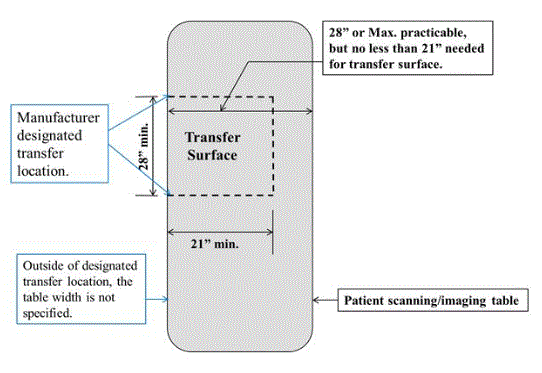
Figure 5.3-9: Schematic of Transfer Surface Size and Patient Support for Diagnostic Imaging Equipment
The following figures show some concepts of accessories to address transfer and positioning supports that could be included an accessibility package.
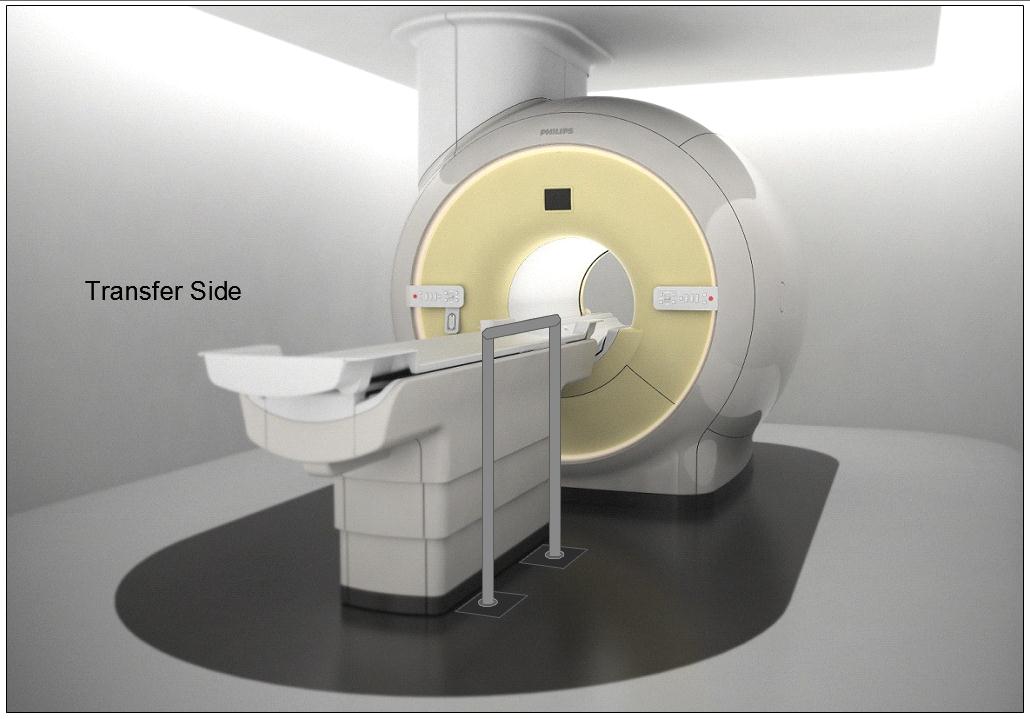
Figure 5.3-10: Illustration of a concept (not to scale) for a detachable floor mounted support. The support could be made to be both height adjustable and detachable at floor level.
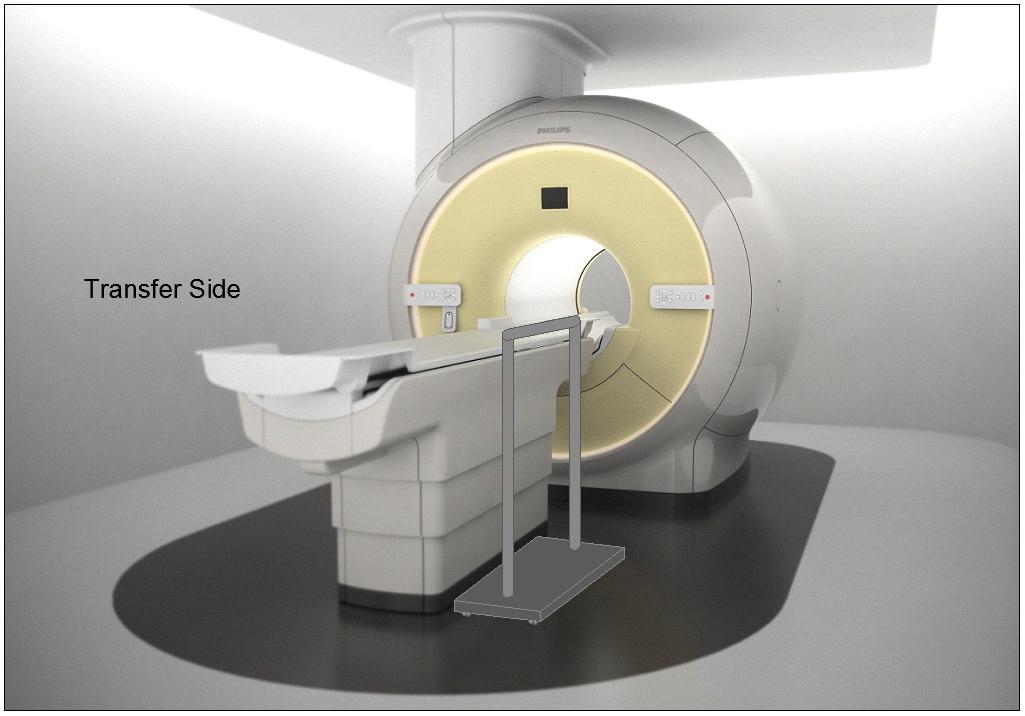
Figure 5.3-11: Illustration of a concept (not to scale) for a wheeled support (wheels would lock and base sufficiently robust and sized for appropriate loadings). The support could be made to be height adjustable.
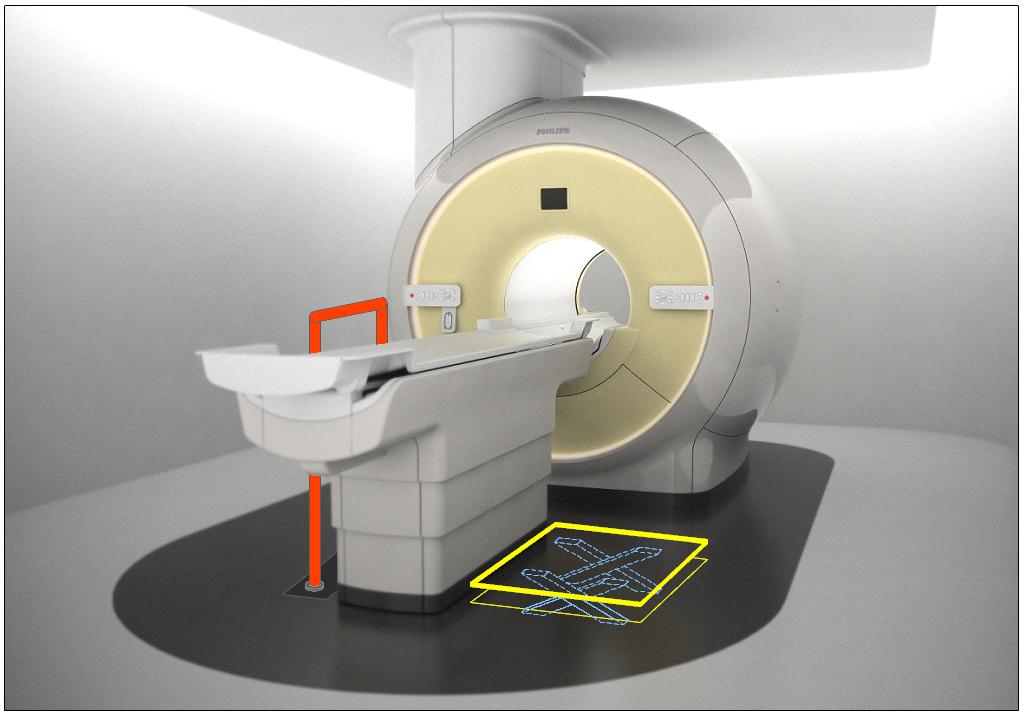
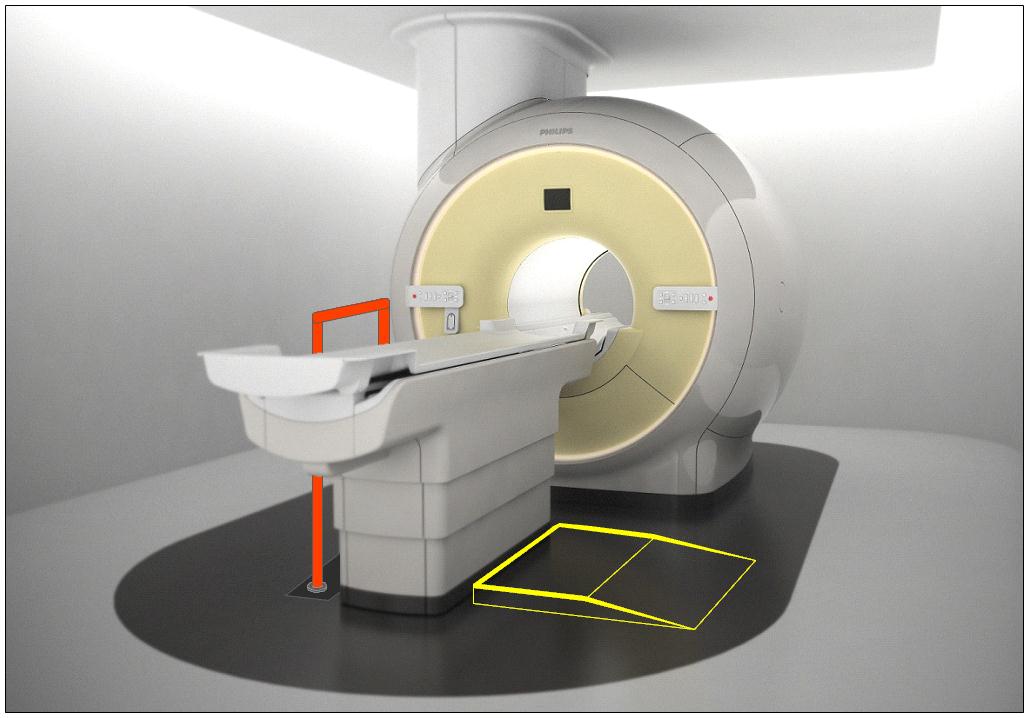
Figure 5.3-12: Illustration of the concept (not to scale) of accessories deployed as part of an accessibility package. The top illustration is shows a floor mounted support combined with scissor lift of Figure 5.3-6. The lower illustration shows the floor mounted support combined with the elevated platform of Figure 5.3-7.
M301.4 Lift Compatibility
Subcommittee Recommendation: (Also agreed to by the full committee.)
An overhead lift (and possibly a gurney-based transfer) may be used in lieu of the provisions for clearances in or around the base of equipment to accommodate the legs of portable floor lifts.
Note: in some circumstances such as in-room MR, the option for a room layout for gurney transfer would be appropriate.
Rationale: The subcommittee recommended the alternate means of using an overhead lift instead of providing clearances for portable lifts because: 1) a ceiling mounted lift can have advantages for better patient transfer and positioning over a portable lift because the portable lift would need to access the diagnostic imaging table from its side or its far foot-end; and 2) table structural design and/or room layout may be such that providing the clearances in and around the base per M301.4.1 and M301.4.2 may be either technically difficult or impractical. Overhead lifts are already currently installed as accessories for some imaging systems.
Overhead lifts are not possible to be used in the MR exam room due to the magnetic fields. Because of this, or for other cases where an overhead lift would be impossible, a gurney-based transfer was considered acceptable provided the gurney met the new standards, even though a double transfer is not ideal.
Below is a picture of an existing CT room with a ceiling mounted overhead lift. Note the degree of flexibility and advantages that this design provides over a portable lift scenario, such as: either side transfer, desired patient orientation prior to transfer, always being available to the patient, no need to manually move the patient by hand, and the ability to move the lift completely out of the way when not needed.
Figure 5.3-13: An existing CT room with a ceiling mounted overhead lift.
6. Transfer Surface Height
6.3 Diagnostic Imaging Equipment
Refer to the Rationale found in Section 5.3 for M301.2.1 and to Section 4.3.
For many types of imaging equipment it will not be feasible to provide a transfer surface meeting the considered minimum height whether it is17, 18, or 19 inches. The same is true for height adjustability for DXA and some X-Ray systems. In those instances an alternate means of access will need to be provided such as the use of auxiliary/ancillary equipment and accessories (“Accessibility Package”).
Given that diagnostic imaging tables are in integral part of the exam and required for accurate results, their design to support very heavy patients, and in some types the existence of imaging components under the table and specialized diagnostic abilities, modifications to the table to go lower and/or be adjustable will always present many design challenges. As such every inch is significant both for designing tables that can go low enough to meet the new standard and in design of accessories or room siting as alternate means to attempt to meet the new standards.
As noted in Section 5.3, the imaging subcommittee did not take a formal position on the transfer surface height. However, during the full committee meeting the manufacturers of diagnostic imaging equipment voted for the “compromise” height of 18 inches.
U.S. Access Board Medical Diagnostic Equipment
Mammography Subcommittee Report:
Final Recommendations on Equipment Criteria
Background: The most frequent type of cancer among women and the second leading cause of cancer-related deaths is breast cancer. A recent study published in the Journal of Women's Health, highlighted that women with disabilities may be less likely to receive a mammogram compared to women without a disability. Prevalence of self-reported mammography use is lower for women with disabilities (72.2% for women 40 years of age or older and 78.1% for women 50 to 74 years of age) than women without a disability (77.8% and 82.6%, respectively), refer to http://www.cdc.gov/features/breastcancerdisabilities/index.html.
Healthcare practitioners find breast-imaging challenging when patients must remain seated in mobility devices such as wheelchairs or scooters for the procedure. In order to obtain a quality image with the needed visualization of the breast tissue, a patient’s ability to approach and position next to the machine is vital. The key equipment barriers arise from the inherent conflict between the mobility device design and the mammography equipment design. The mammography device operation seems designed to require the patient has the ability to stand. Therefore, a woman using a wheelchair will experience two main barriers, the first is that her mobility device cannot get close enough to the mammography machine itself, and secondly, even if she does get close enough to the machine, the breast platform height does not lower enough to image a woman’s breasts while in the seated position.
The Mammography Subcommittee members provided varied expertise. Members included representation from disability advocates, healthcare organizations including technologists, industry designers, and engineers. Industry input provided supporting illustrations, which enhanced the discussion.
Overview: The subcommittee recognized that to get the best accessibility, equipment would need redesign to accommodate persons seated in wheeled mobility devices for the exam. In developing its proposals, the subcommittee considered the physical constraints needed for operation of the equipment, technical feasibility, patient safety, and clinical standards. The subcommittee weighed all the provisions for technical criteria in light of the equipment functionality and the need to image a range of body sizes, functionality, and types.
The subcommittee considered the interaction of mammography devices with individuals using scooter. Due to the need for the woman’s chest wall to be flush with the breast platform, the front scooter components make this position impossible without the person swiveling to the side. Thus, the final recommendations focused on the mammography device’s interaction with wheelchairs. Mammography equipment recommendations are also operable with use of a mammography chair for patients who need to sit for the procedure but do not use a wheelchair.
Mammography imaging involves complex interactions between moving parts. To get the final dimensions, the subcommittee needed to consider the elements “dynamically.” The dynamic angle is necessary because of the way the equipment operates. Recognizing that all operations center on the movable breast platform, recommendations define each component in relationship to the breast platform. The unique aspects of mammography device design and operation requires components to work when the breast platform is at breast height. Since breast platform design affects most of the other device components, defining each in relationship to the breast platform creates proportions that when incorporated into the design process assure that women in mobility devices can get effective imaging.
Ordinarily, the accessibility dimensions are static and measured from a static element such as the floor. Because of the movable breast platform and C-arm, each recommendation defines the other device components in relation to the breast platform. The static point is with the breast platform at the height of 34 inches. This allowed the subcommittee with industry input to consider and maximize the dimensions that improved the accessibility during the equipment’s operation. The result is that this report defines traditional accessibility elements from a different vantage point.
To determine the final criteria for the mammography devices, the subcommittee balanced the knowledge of disability, range of physical characteristics and diversity of body types and sizes with each device component. The members considered industry input on how the dimensions and operation of one element would interact with the other elements. Such input helped interpret how these interactions would affect each other. The subcommittee used the best data available recognizing that the data available did not always match precisely the operations of mammography equipment.
To determine the recommendation, it was critical for all to have an understanding of the equipment components. The diagram below illustrates the set of terminology and the location on a mammography device used to describing the mammography components below. (Figure 1 below)
Figure 1. Overview of the subcomponents of a mammography machine.
In order to get a quality image, the patient’s breast needs to rest on top of the breast platform and the chest wall needs to be flush with the front edge of the breast platform. For this to be possible, the breast platform needs to go low enough to accommodate a patient seated in a wheelchair. There also needs to be enough knee and toe clearance to ensure that the patient can get close enough to the breast platform without knees or feet hitting parts of the equipment. One important feature of mammography equipment is the base support, also shown in Figure 1, which is critical for structural support, seismic stability, and installation safety. This base support must be low enough so that a patient’s footrests can ride over it and it must allow enough unobstructed floor space to ensure that a wheelchair’s front caster wheels do not hit it. Lastly, the configuration of the positioning supports must provide enough flexibility for all patients to be able to reach and hold them. An illustration of each is in Figure 2 below.
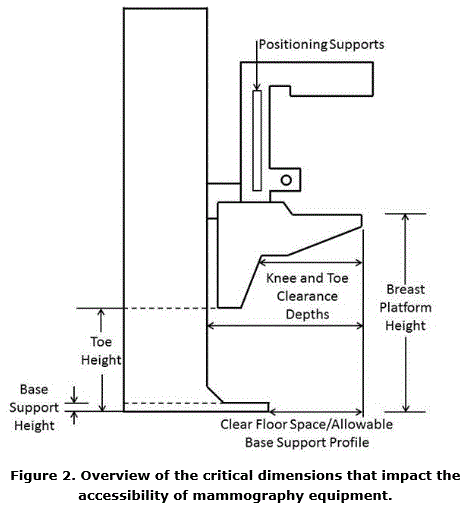
Figure 2. Overview of the critical dimensions that impact the accessibility of mammography equipment.
Breast Platform [M303.4.1]
Recommendation: The final recommendation for the minimum height of the breast platform is 26 inches from the floor to the top of the breast platform. The upper height range for the breast platform was not controversial and remained as proposed except for the designation of this as a minimum upper height. The final version of the criteria must clarify that there is no need to limit the height of the breast platform since most are already above the 42-inch measurement at the top end.
Rationale: The subcommittee expressed conflicting perspectives on the minimum breast platform height with members either choosing to support the 26-inch or 28-inch minimum. While all other subcommittee decisions were by group consensus, the group required a formal vote on this issue. Although the subcommittee vote was for 28 inches, during the final public meeting, the full committee supported the 26-inch minimum by a strong majority.
According to industry, equipment currently manufactured ranges anywhere between 25 and 28 inches for the lowest measurement of the breast platform. There were various reasons cited for each of the positions. Recommendations from accessibility experts who developed mammography protocols for women with disabilities identified a need for a breast platform height of 24 inches. Because this recommendation evolved from technologist experience on equipment with less knee space, disability advocates supported the rationale for 26 inches as the minimum. One member cited the diversity of body types and sizes for persons with disabilities as the rationale for the 26 inches. Another member emphasized the importance of considering patients of short stature in addition to considering patients seated in a wheelchair.
Many industry organizations supported the 28-inch minimum. Reasons cited included providing more flexibility for manufacturers and concern that the lower minimum could result in more leg injuries as the technologist lowered the breast platform so close to the lap of the patient using a wheelchair. Informal input from some technologists indicated no problems with imaging patients with disabilities at 28-inch minimum heights. Members disagreed with limiting the height as the method of minimizing the risk of injury since this could happen at any height depending upon the height of the knees of the person.
One member expressed support for the lower height in order to facilitate the Magnified Cranio Caudal (hereinafter “Mag”) view for person needing magnified views of tissue. Mag views require the breast to be located at a specific position in between the x-ray tube and the breast platform. To capture this view, providers place an accessory, often called a “Mag Stand”, on the breast platform, as shown in Figure 3 below. The provider will then position the patient’s breast on top of the mag stand so that the breast is at the correct position between the x-ray tube and the breast platform. The height of the mag stand can vary between 6” and 10”, depending on the characteristics of the x-ray beam and the degree of magnification desired. Since the technologist places this accessory on top of the breast platform, it effectively increases the height and thickness of the breast platform. Beginning with a lower breast platform height could help more patients to have access to the mag view. However, the more significant barrier to patients imaged from a seated position is the increased overall size of the platform (from lap to breast) during a mag view, since it would require the patient to have a very tall torso.
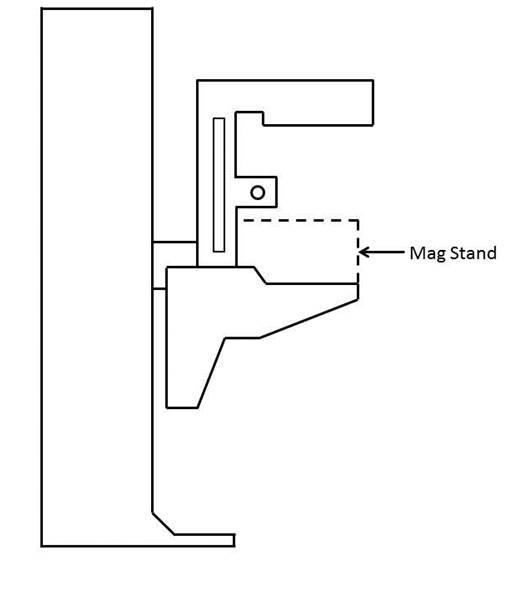
Figure 3. Diagram of a Mag Stand positioning on the breast platform.
Knee & Toe Space [M303.2.4]
Recommendation: The final subcommittee’s recommendations increased the overall knee and toe space to a minimum of 28 inches, from the initially proposed 25-inch absolute dimension. The final recommendation for knee clearance directly underneath the breast platform is a minimum of 18 inches and the final recommendation for the unobstructed floor space in front of the base support is a minimum of 17 inches. Each of these measurements creates a more accessible piece of imaging equipment balanced with the physical attributes and operation of the equipment.
Rationale
The issue of Clearance and Unobstructed Floor Space
The knee and toe clearance dimensions, as well as the unobstructed floor space dimensions, dictate how close a patient in a wheelchair can get to the mammography equipment. The initial NPRM dimensions used information for the clear space under a desk or table as the basis for the proposed regulation. Industry suggested the dimension might be different because mammography procedures require a person’s chest to be flush with the front of the breast platform. The subcommittee reviewed the existing data of wheelchair users1 and industry input on how these elements technically affect one another. Below is an overview of the rationale for each of the final recommended dimensions affecting clearances in front of the gantry. (Table 1)
Table 1. Critical Equipment Dimensions with the relevant Anthropomorphic Data
| Critical Dimension | Anthropomorphic Data /Rationale | Final Decision |
| Height of Breast Platform At Time of Measurement | Set point for assuring knee clearance at 27” above the ground Assures proportionality of each element to each other |
34" |
| Knee Clearance Depth at 27” above the ground | 95th percentile knee clearance measured from abdomen to front of person’s knees, female-only data | 18" minimum |
| Clearance Depth at Toe Height above the ground | 95th percentile knee clearance measured from abdomen to front of person’s legs at toe height above the ground, combined male and female data due to availability | 22" minimum |
| Toe Height | 95th percentile toe height, female-only data; consideration of breast platform moving to lower heights | 16" minimum |
| Overall Knee and Toe Clearance | Abdomen-to-toe depth, female only data; technical information; consideration of positioning methods | 28" minimum |
| Unobstructed Floor Space | Abdomen-to-caster wheel depth, combined male and female; technical information; consideration of positioning methods | 17" minimum |
One issue affecting how close the patient can get to the mammography machine is the base support, an essential equipment element that intrudes into the floor space. As discussed below, the purpose of the base support is to eliminate any instability caused by the weight of the cantilevered c-arm. Moving the c-arm further away from the gantry would increase the overall knee and toe clearance, but would also require an increase in the size of the base support in order to stabilize the equipment. Since the base support obstructs the floor space, resulting gains from increasing the knee and toe clearance are minimized because of the simultaneous increase in the size of the base support. The subcommittee considered this balance when making final decisions about overall knee and toe space and unobstructed floor space.
While discussing these issues, the subcommittee also considered positioning methods for patients using wheelchairs. During a mammography procedure, the patient’s chest is flush with the front edge of the breast platform and the technician positions the patient’s breast on the breast platform. In order to image as much of the breast as possible, patients cannot be seated in the “natural” sitting position. They will need to sit up straight in order to situate as much breast tissue as possible on the breast platform. For patients seated in a wheelchair, a common positioning technique is to place towels, pillows, or other positioning aids behind the patient’s back in order to sustain an upright pose during imaging. This upright pose is necessary regardless of how close the patient can naturally get to the breast platform. The pose reduces the abdomen-to-toe and abdomen-to-caster edge dimensions by a couple inches. The anthropomorphic data shows that 30.5 inches and 19.5 inches are the needed overall knee and toe clearance and unobstructed floor space dimensions, respectively. (Data shown below in Tables 2 and 3).
Table 2. Abdomen Depth Data to determine overall knee and toe clearance
| Percentile Value | |||||
| User Group | Median – 50th | 75th | 90th | 95th | Max |
| Female Manual Wheelchair Users (n=130) | 23.2 | 26.4 | 29.2 | 30.6 | 33.0 |
| Female Power Wheelchair Users (n=89) | 21.6 | 24.8 | 28.1 | 30.4 | 36.7 |
Table 3. Abdomen-Caster Edge Depth
| Percentile Value | |||||||
| User Group | Min | 5th | 10th | 50th | 90th | 95th | Max |
| Manual Wheelchair Users (n=101) | 2.0 | 5.6 | 6.8 | 11.4 | 17.1 | 18.5 | 20.3 |
| Power Wheelchair Users (n=67) | 4.1 | 6.0 | 7.8 | 10.9 | 17.6 | 19.3 | 21.9 |
In applying the various positioning considerations along with the data, measurements of 28 inches (full knee and toe clearance above the base support) and 17 inches (clearance underneath the breast platform) would capture nearly everyone. Looking purely at the anthropomorphic data above, these dimensions accommodate ~90% of the population. In adjusting for the needed upright pose, an adjustment of at least 2.5 inches is likely. If so, the equipment accommodates 95% of the population since the difference is ~2.5 inches less than the 95th percentile of the corresponding anthropomorphic dimensions. Members recognized that these dimensions are an optimal blend of accessibility and technical feasibility2 when balanced with the physical design and operation of the equipment,
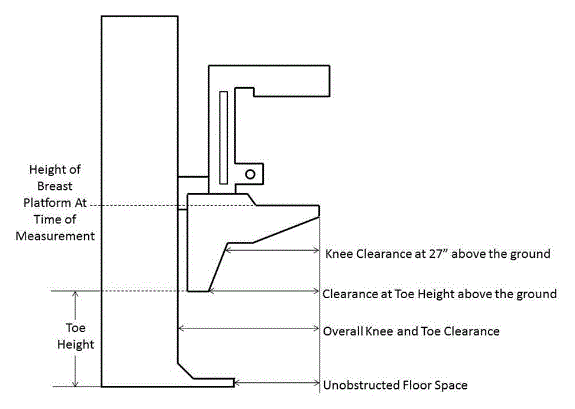
Figure 4. Overview of the critical dimensions that define the knee and toe space underneath the breast platform.
Relationship of knee and toe space with breast platform
The shape of the breast platform affects the shape of the knee and toe space. Since the shape of the breast platform varies across different manufacturers and equipment, merely defining the knee clearance as the “knee clearance below the breast platform” would lose the interrelated aspect of these elements. This means a particular feature on the breast platform does not define the required location of the knee clearance. Instead, the knee space dimension is located with the platform at a specified height. By defining the height of the breast platform as the “set point” for the other dimensions, all clearances have a static measurement point that assures maximum clearances during operation of the equipment. Ultimately, the knee dimensions must be determined when the person’s breasts are on top of the breast platform and there must be sufficient clearance at the height of the person’s knees, as is shown in Figure 6 below. By using this approach, the subcommittee was able to maximize the clearances in relationship to the equipment operations.3
The NPRM proposed measuring the knee clearance under the breast platform at 27 inches above the ground. Industry used this dimension and determined the proportionate height of the breast platform at a height of 34 inches. The subcommittee agreed to this as a “set point” (Identified as “Breast Platform Height” in Figure 5 below). Thus, when the patient’s breast is on the breast platform, there must be sufficient knee clearance at 27 inches above the ground (“Knee Height” in Figure 5 below). These dimensions (27 and 34-inch heights) are the same as is defined by the current ADA/ABA accessibility standard to accommodate wheelchair users at desks. The dotted lines in Figure 6 below illustrates this dimension.
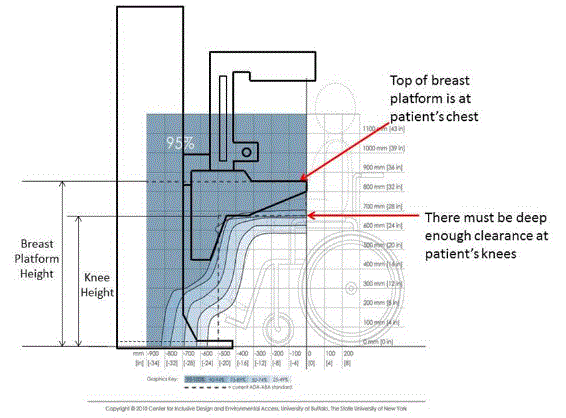
Figure 5. Using the height of the top of the breast platform as a set point for the knee and toe clearance measurements.
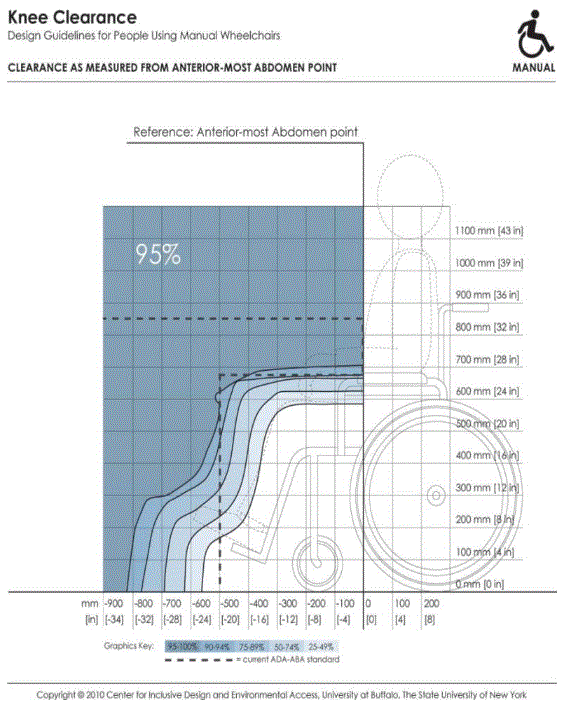
Figure 6. Illustration of current ADA/ABA standard to accommodate wheelchair users sitting at desks.
The two dimensions define the breast platform requirements for a person with the deep knees and a tall wheelchair. When the breast platform lowers to accommodate shorter patients in wheelchairs, there will be ample knee clearance for those patients. Conversely, as the breast platform moves up for patients who have longer torsos, the breast platform will just be moving away from their knees and legs, thus giving them ample space as well.
Toe Height
The NPRM defined the toe clearance at a height of 9 inches. However, as the breast platform moves up and down, it may intrude on the toe space. The subcommittee recommended that the toe height be set to 16 inches. Looking further at female-specific toe height data, the 95th percentile toe height was 13 inches for manual wheelchair users and 16.1 inches for power wheelchair users. As such, the subcommittee recommended that the toe height be set to 16 inches (when the breast platform is at the 34 inch height discussed above). This higher 16-inch measurement (compared to the originally proposed 9-inch toe height) would also minimize the chance of the breast platform hitting patients’ toes when the breast platform moves to some of its lower heights
Again, normally a toe space dimension defines a static unobstructed space. In this case, in order to get the maximum accessibility out of all the dimensions, the final recommendation requires the dimension of toe clearance is determined at the 34-inch breast platform height (see discussion above). This higher 16 inches measurement (compared to the originally proposed 9 inches toe height) would minimize the chance of the breast platform hitting patients’ toes when the breast platform moves to some of its lower heights. Providing this dimension helps define the outer limits of the breast platform configuration in a way that maximizes the dimensions for accessibility.
When considering toe height, it is more convenient to use the anthropomorphic model that defines toe height specifically, shown below in Figure 7 for manual chairs.
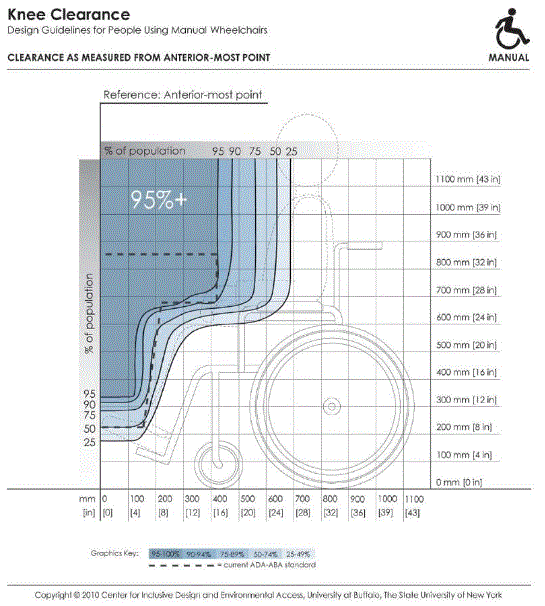
Figure 7. Anthropomorphic model used to analyze toe height.
If the toe height is at 16 inches above the ground when the breast platform is at 34 inches above the ground, we are accommodating the 90-95th percentile person. The toe height is more than enough to accommodate wheelchair users since the 95th percentile toe height is 13 inches. When the breast platform lowers to a 26-inch height, then the toe clearance height would be 8 inches. This maintains proportions likely to accommodate persons needing the breast platform at the lowest height since knee height is also less.
As discussed above, the minimum range of travel for the breast platform is 26 inches to 42 inches. When the top of the breast platform is at its lowest position of 26 inches, there is no need for knee clearance at 27 inches above the ground (knees will be lower than breast height). Defining knee and toe clearances based the lowest height breast platform at 26 inches may compromise taller patients in higher wheelchairs. As such, it is more appropriate to measure the dimensions of the knee and toe space set the breast platform to a higher height to ensure that the greatest required knee and toe clearances. Then, when the breast platform lowers to accommodate smaller patients, there will be more than enough clearance available.
The diagram below illustrates the final knee and toe clearance recommendations (Figure 8).
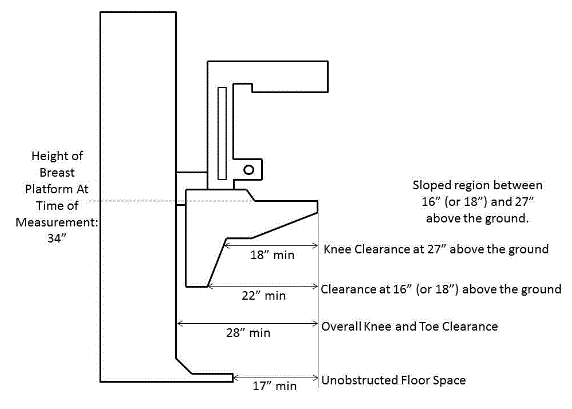
Figure 8. Summary of knee and toe clearance recommendations.
Notes:
1. Where possible, the subcommittee focused only on the anthropomorphic data of women, the primary group needing mammography.
2. The concept of technical feasibility is one found in other areas of access. It recognizes that there may be physical factors that make create limits to what functions can be achieved even with redesign. In mammography, the physical stability and operation of the equipment fall into this category. The committee recognizes that future visionaries may be able to “re-vision” new devices.
3. Given the differing shapes of mammography devices, defining the dimensions in relationship to each other with a static measurement point for the breast platform assures accessibility despite the varied shapes and dimensions of the breast platform. Otherwise,, it is quite possible to configure a piece of equipment that meets all the clearances yet does not provide accessibility if there is not a defined method of measuring the clearances in relationship to the breast platform.
4. Measured from the point on the floor where a perpendicular line intersects the front edge of breast platform shown in Figure 10.
Base Support [No specific provision—affects M303.2.4]
Recommendation: The final proposal on the base support is for 1½ inch high maximum base support with additional sloping permitted at 25 inches or beyond4 to the gantry if needed for additional structural support.
Rationale
The base support is of fundamental importance to mammography equipment and provides structural support, seismic stability, and installation safety. It does obstruct the floor space in front of the gantry and, thus, may limit how close a wheelchair can get to the equipment. To respond to this issue, industry proposed a configuration that would allow wheelchair footrests to ride over it so it causes minimal obstruction to the floor space in front of the gantry. To discuss the maximum base support height, the sub-committee looked at anthropomorphic data regarding footrest heights. The footrest height data measures the height from the floor to the top surface of the footrest at its proximal outside corner. To determine the necessary clearance for the footrests, the subcommittee used the calculated thickness of the footrests (~0.5 inch) minus the footrest height measurement. The ~0.5” thickness is the recommendation from the data supplied by Steinfeld and D’Souza. The anthropomorphic data, as well as the calculated footrest clearances, are below in Tables 4 and 5.
Table 4. Anthropomorphic data on measured footrest heights
| Device Type | Min | 5% | 10% | 50% | 90% | 95% | Max |
| Manual (n=101) | 1.2 | 1.7 | 2.2 | 4.1 | 7.2 | 10.0 | 36.2 |
| Power (n=67) | 1.8 | 2.7 | 3.1 | 4.8 | 8.8 | 10.5 | 16.9 |
Table 5. Actual footrest height clearances
| Device Type | Min | 5% | 10% | 50% | 90% | 95% | Max |
| Manual (n=101) | 0.7 | 1.2 | 1.7 | 3.6 | 6.7 | 9.5 | 35.7 |
| Power (n=67) | 1.3 | 2.2 | 2.6 | 4.3 | 8.3 | 10 | 16.4 |
A maximum base support height of 1.5 inches will provide room for the structural components necessary for an effective base support design and will also be accessible by ~92% of manual chair users and over 95% of power chair users.
The subcommittee also discussed adding an allowance for a sloped region at the base of the gantry. Many of the mammography machines today also have important informational displays at the base of the gantry, on top of the base support (shown as the checkered region in Figure 9 below) that display information such as compression force and compression thickness.
Industry input on these displays cited customer feedback. The technologists felt that while positioning the patient’s breast, they were naturally looking downward, and by having the display at the base of the gantry, they could watch the compression force and breast thickness readout while positioning the patient. They suggested this location for the display as a method for improving workflow. On other machines, this region has other mechanical and electrical components that are important to the function of the equipment.
At the full committee meeting, there was extensive discussion regarding the additional sloped region. Several members felt strongly that the additional sloped region must be justified by structural stability since displays could be located elsewhere. Members commented that not all equipment has displays in this region, so it seemed possible for industry to redesign the displays to create less interference.
The subcommittee agreed on adding an allowance for a sloped region as defined by Figure 10 below. Figure 11 below illustrates this region overlaid with the anthropomorphic data.
Figure 9. Location of the display area at the base of many mammography machines.
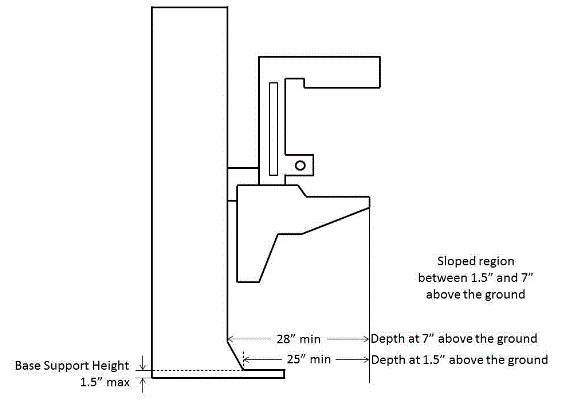
Figure 10. Summary of base support recommendations.
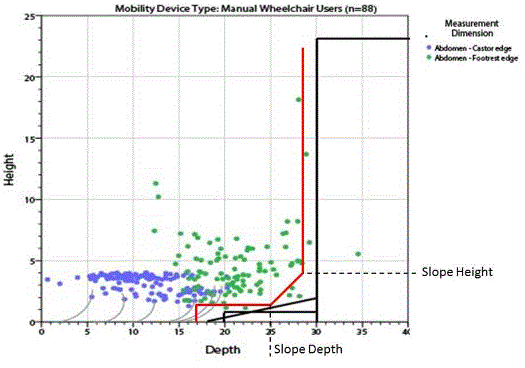
Figure 11. Base support configuration in red shown over the anthropomorphic data.
Steinfeld and D’Souza supplied this chart plotting their study’s findings on the depth of participant’s footrests. The black lines show the areas with no interference between the footrests and base support interface. The black lines illustrate areas where no footrests would intrude. However, based on the technical feasibility and clinical use considerations discussed in the above sections, industry provided the overlay configuration in red. The base support configuration illustrated above is the final recommendation.
The green dots in the above scatter plot illustration represent observed values for footrest height, (on the vertical axis) and footrest depth (on the horizontal axis) measured for manual wheelchair users to the abdomen point. The footrest clearances (calculated in Table 5 above) are ~0.5” lower than the footrest heights defined here. The footrest depth is the horizontal distance from the abdomen to the distal (forward-most) edge of the footrest. The subcommittee used the data for manual chairs because manual chairs are lower than the power chairs.
Summary Figure: Figure 12 shows the final recommended component dimensions in one diagram.
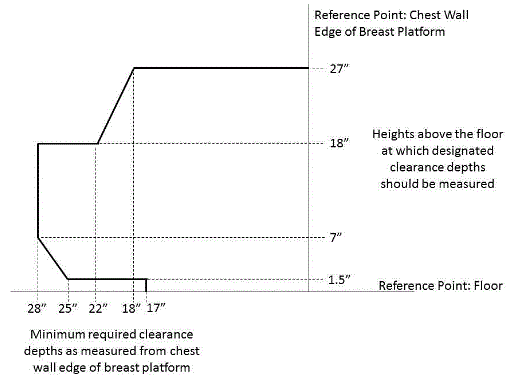
Figure 12. Summary of final clearance recommendations, to be measured when the top of the breast platform is at 34”.
Notes:
4. Measured from the point on the floor where a perpendicular line intersects the front edge of breast platform shown in Figure 10.
Standing Supports [M305.2]
Recommendation: The final recommendation of the subcommittee is to remove the requirement for standing supports in reference to mammography equipment. Instead, positioning supports should be required. To be useful, positioning supports must shaped for grasping, positioned within the reach range of all users, and be 12 inches long minimum when located on the moving c-arm or 18 inches long minimum when in a fixed location.
Rationale
The original criteria proposed in the NPRM included vertical standing supports on both sides of the equipment that were 18 inches in length. During discussion, there was general agreement that the real use of these supports is for positioning, and thus, not meant to support the full weight of the patient or to keep a patient in a standing position during the exam. Industry representatives posited that if a patient has limitations or balance issues severe enough to need standing assistance, then the healthcare provider should position her in a seated position for safe imaging. The primary use of these supports is for positioning of the arms during the imaging process to keep them out of the field of view of the image. For these purposes, the equipment criteria should designate these supports as positioning supports and be designed with the structural strength required for positioning activities.
Furthermore, since many types of mammography equipment mount the supports on the c-arm, they will move up and down with the breast platform. In these cases, they do not need to be as long as 18 inches to provide sufficient flexibility for patients to reach them. Industry representatives also indicated that there are controls in the area where these positioning supports are located. It is important that the patients’ hands do not accidentally hit these controls when they are holding the positioning supports. For this reason, industry will sometimes intentionally shorten the length of these handholds to less than the 18-inch proposal. Considering these factors, the full committee agreed that a 12-inch long positioning support would be sufficient if it moved with the movable breast platform.
Mammography Chair [M302]
Recommendation: The mammography chair is an essential piece of equipment allowing persons who are unable to stand or balance to sit for the diagnostic procedure. The chair must meet the elements of M302 with the additional requirement that any footrests must accommodate and ride over the base support.
References:
The following sources were referenced for the anthropomorphic data presented here. Specific citations are made in the full committee report.
IDeA Center Study at the University of Buffalo, New York: Summary analysis of wheelbase measurements on wheeled mobility devices.
IDeA Center at the University of Buffalo, New York: The Wheeled Mobility Anthropometry Project, Final Report, pages 89-92.
D’Souza, et al. “Clearance Space Envelopes of Wheeled Mobility Device Users for Computer Workstations”. Proceedings of the Human Factors and Ergonomics Society Annual Meeting 2012.
D’Souza, Steinfeld. “Revised Knee Clearance Depth and Footrest Clearance dimensions for sizing of mammography equipment.” Memorandum. April 23, 2013.
Notes:
1. Where possible, the subcommittee focused only on the anthropomorphic data of women, the primary group needing mammography.
2. The concept of technical feasibility is one found in other areas of access. It recognizes that there may be physical factors that make create limits to what functions can be achieved even with redesign. In mammography, the physical stability and operation of the equipment fall into this category. The committee recognizes that future visionaries may be able to “re-vision” new devices.
3. Given the differing shapes of mammography devices, defining the dimensions in relationship to each other with a static measurement point for the breast platform assures accessibility despite the varied shapes and dimensions of the breast platform. Otherwise,, it is quite possible to configure a piece of equipment that meets all the clearances yet does not provide accessibility if there is not a defined method of measuring the clearances in relationship to the breast platform.
4. Measured from the point on the floor where a perpendicular line intersects the front edge of breast platform shown in Figure 10.
Medical Diagnostic Equipment Accessibility Standards
Sub Committee Recommendations
Stretcher Final Report
May 31, 2013
The Subcommittee’s technical recommendations for the stretchers have taken into consideration the following technical criteria proposed in Chapter 3, part 1195 to Title 36 of the Code of Federal Regulations. Specifically, Section M301, Diagnostic Equipment Used by Patients in Supine, Prone, or Side-Lying Position.
Equipment Evaluated: Stretchers
Stretchers are typical equipment used in healthcare settings for patients in supine, prone, or side-lying positions.
Chart from Proposed Accessibility Standards for Medical Diagnostics Equipment Appendix to part 1195
| Patient Positions Equipment Designed to Support | Equipment Features Addressed in Technical Criteria | Types Of Equipment To Which Technical Criteria Applies |
| Supine, prone, or side-lying position (M301) | Transfer surface, including height, size, and transfer sides Transfer supports, stirrups, and head and back support Lift compatibility |
Stretchers |
| Seated Position (M302) | Transfer surface, including height, size, and transfer sides Transfer supports, stirrups, and head and back support Lift compatibility |
Stretcher chairs |
Equipment Evaluated: Stretchers
Sub-Committee Summary of Scope for Recommendations
The Full Committee and Sub-Committee are both in agreement that Beds are excluded from these proposed standards as beds are used for therapeutic and not diagnostic procedures. Stretchers are used not only as transport devices but are heavily used in the healthcare setting to perform diagnosis.
M301.2.1, M302.2.1 Transfer Height
Proposed requirement:
The height of the transfer surface during patient transfer shall be 17 inches (430 mm) minimum and 19 inches (485 mm) maximum measured from the floor to the top of the transfer surface.
• Sub-Committee Recommendation: 17 inches with exceptions.
Exception: During patient transfer, the height of the transfer surface shall have a lower height of 21" maximum and adjustable to a higher height of 25" minimum, measured from the floor to the uncompressed height of the patient support surface.
Rationale: Early studies are showing that achieving a low height for stretchers in the 17"-19" range to be very difficult due to the various roles of stretchers being not only diagnostic pieces of equipment, but as that they are used for transport. The very nature of the mobility systems and components of the stretcher configuration, including wheels, brakes and steering systems as well as their ability to carry portable equipment such as IV's, infusion pumps, monitors, oxygen, foley catheter bags, etc. all of which create serious packaging constraints that limit the low height of the patient support surface.
Patients may utilize stretchers for long periods of time – e.g. emergency room (primary equipment used) or while waiting to have their diagnostic tests performed. Stretchers are also widely used in outpatient surgery centers. Other considerations for stretchers are the surfaces (mattress) that is purchased separate from the stretcher deck. The Standard of Care for healthcare organizations is to have a pressure-relieving surface on the stretcher to help prevent pressure ulcers. These surfaces may range between 4” to 5” in thickness - this will add to the vertical height of a stretcher when measuring from the floor to the top of the surface (uncompressed). The Committee acknowledges this Standard of Care and feels it is important to maintain when measuring overall the height. Stretchers currently have a low deck of 20” – 23”.
This is also coupled with the complication of a 6" tall lift clearance window that forces foot operated controls up. These elements are all competing for the same vertical space and it will take full development of the stretcher to confirm or deny the ability to meet the desired range of 17"-19". Until that time, it is proposed we take a more conservative position and push the low height slightly higher for stretchers until the technology can catch up with the regulations and full feasibility studies can be researched. The recommendation for exception is 21" (with a oxygen holder). When access is unachievable, a portable floor or ceiling lift shall be utilized.
Figure 1: Stretcher Vertical Height Constraints – Configuration of Features
(Picture provided by Hill-Rom)
Note: In the healthcare setting having a single stretcher type that services a majority of patient conditions is desired. Patients may use stretchers for transport only, however many patients may also require supporting oxygen during transport to and from diagnostic procedures.
Figure 2: View of Stretcher with Oxygen Tank
Note: Oxygen tanks require a minimum of 5” of vertical height in stretcher configuration
(Picture provided by Stryker)
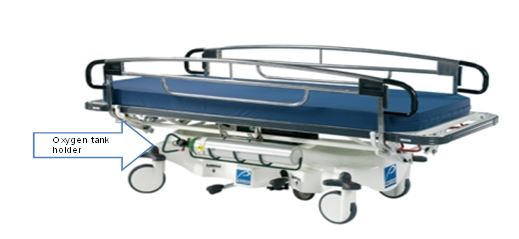
M301.2.2, M302.2.2 Transfer Size
Proposed requirement:
30" wide minimum and 15" deep minimum
• Sub-Committee Recommendation:
28" wide minimum and 17" deep minimum. This transfer surface is located on the longitudinal (long) side of the stretcher—either right or left side
Rationale: Based upon full committee discussion and harmonization with Exam Table Subcommittee.
M301.2.3 Transfer Sides
Proposed requirement:
The transfer surface shall be located to provide options to transfer from a mobility device onto one short side (depth) and one long side (width) of the surface.
• Sub-Committee Recommendation:
The transfer surface shall be located to provide options to transfer from a mobility device onto both long sides (length), right and left, of the surface.
Rationale: Revision is based upon the configuration and use of stretchers. The configuration of stretchers will not allow for unobstructed access to a transfer surface located at either the foot end or head. The location of the Transfer Side will be located on the patient right or patient left at roughly midway between the foot end and head end. This places the position at a desirable location along the length of the stretcher side so as when the transfer is completed, the patient is in the correct location relative to the articulation of the backrest. As a result, a patient can only access one long side of the transfer surface, the one short side will be inaccessible.
Proposed requirement:
Each transfer side shall provide unobstructed access to the transfer surface.
• Sub-Committee Recommendation:
Each transfer side shall minimize obstructions to the transfer surface. Obstructions shall not be vertically less than 1" (25 mm) nor be horizontally more than 3" (75 mm) from the edge of the patient support surface provide unobstructed access to the transfer surface.
Rationale: The position of obstructions has been discussed by the full committee and the Exam Table subcommittee and has established an obstruction protruding no more than 3" beyond the edge of the transfer surface.
It is proposed herein that an additional requirement be added to restrict how high the obstruction can exist in comparison to the top of the transfer surface. Guidance from IEC 60601-2-52 does establish the maximum vertical obstruction of being not less than 1" below the top of the transfer surface.
M301.3 Transfer Supports
Proposed requirement:
Transfer supports shall be provided for use with the transfer sides required by M301.2.3 and shall comply with M305.2.
• Sub-Committee Recommendation:
No revision.
* Other Transfer Support Dimensions: (*not included in the Proposed Rule)
• Sub-committee Recommendation:
Length - At least 15 inches (300 mm) in length and located on the length of the transfer surface. Located on the opposite side of the access point.
Circular cross sections – Circular cross sections must have an outside diameter of 1¼ inches minimum and 2 inches maximum. Section may be discontinuous (see Rationale) along the length of the Gripping Surface not exceeding 20% of the length of the gripping surface.
o Rationale: Discontinuous allows for the vertical support structure that is necessary for supporting the grip.
Non-circular cross sections - Non-circular cross sections must have a cross section dimension of 2 inches maximum and a perimeter dimension of 4 inches minimum and 4.8 inches maximum. Section may be discontinuous* along the length of the Gripping Surface not exceeding 20% of the length of the gripping surface.
Transfer Supports Position
• Sub-Committee Recommendation:
Located longitudinally within the long side of the of the transfer surface. Transfer support will be located on the opposite side of the transfer side.
Length: Minimum of 15" of continuous support.
o Rationale: This is to accommodate the articulation that is necessary for the head and back support on stretchers.
Height: Located no less than 6 inches (150 mm) and no more than 19 inches (482 mm) above the patient support surface
Horizontal Distance from Transfer Surface: No more than 3 inches (75 mm) from the edge of the patient support surface.
o Rationale: Currently, and it is envisioned that transfer supports will continue to be enabled through the side rail systems currently employed on stretchers. Based on the nature of side rail mechanisms to allow them to fold and store properly. Currently, the state of the art for side rail have them placed just under 3" outboard of the mattress surface edge (transfer surface edge). Therefore, it is requested that this horizontal dimension allow for current designs to fall under the proposed rule.
Figure 3: Transfer Support Location – Plan View
(Drawings designef for Access Board by Stryker)
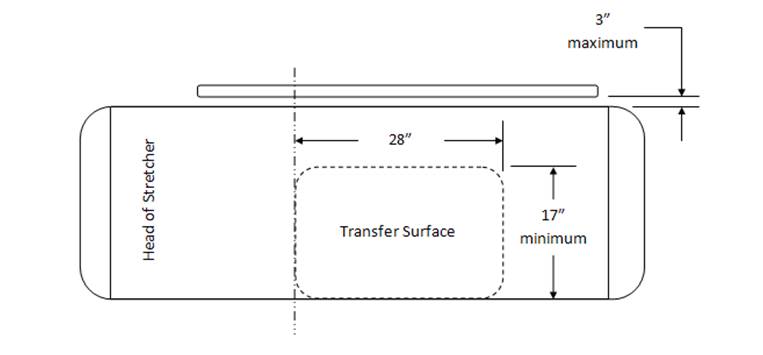
Figure 4: Transfer Support Location – Side View
(Drawings designef for Access Board by Stryker)
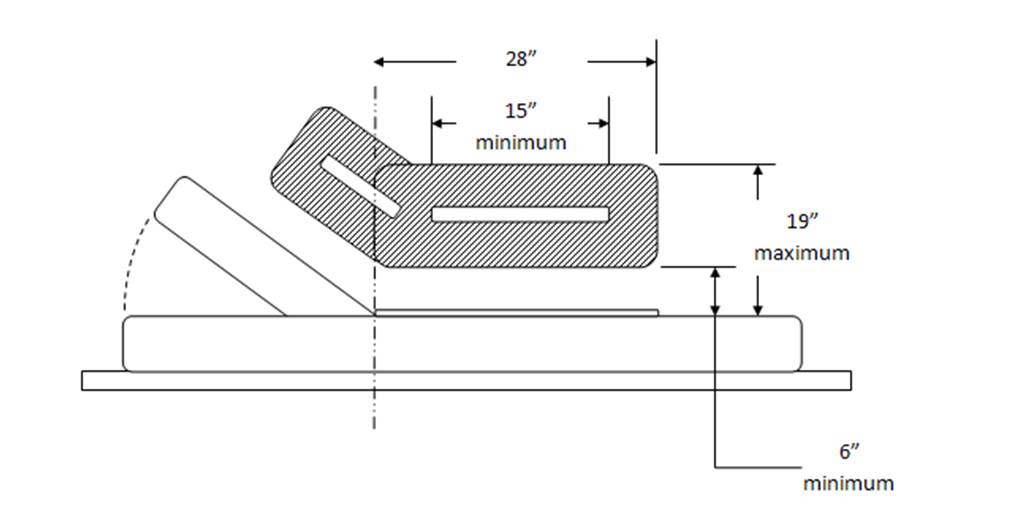
M301.3.2 Stirrups
Proposed requirement:
Where stirrups are provided, they shall provide a method of supporting, positioning, and securing the patient’s legs.
• Sub-committee recommendation:
Where stirrups are provided, a method shall be provided for supporting, positioning, and securing the patient’s legs.
Rationale: Stirrups (also called foot supports) alone may not provide a method of supporting, positioning, and securing the patients legs, but may be supplemented by or used in conjunction with a secondary accessory that provides this function. Typical accessory is a set of calf supports that support the patient legs just below the knee. Recommending a slight revision to this wording. When stirrups are provided "A" method shall be provided for supporting, positioning and securing the patient's legs. This wording change allows for alternate methods to be used for this repositioning, etc Full Sub-committee approval for this change.
M301.3.3 Head and Back Support
Proposed requirement:
Where diagnostic equipment used by patients in a supine, prone, or side-lying position, and diagnostic equipment used by patients in a seated position can be adjusted to reclined positions (M301.3.3 and M302.3.3) would require head and back support to be provided throughout the entire range of the incline. This is consistent with ANSI/AAMI HE 75 which recommends that the “support surface needs to be adjustable or have adjustable features (e.g., for the head, neck, back, lumbar region, leg, knees, and foot, as appropriate) to support patients in various postures and body positions in a manner that optimizes their comfort. See ANSI/AAMI HE 75, section 16.4.7 (h. Although not required by the proposed standards, examination tables that can be adjusted to a sitting position and then reclined to a horizontal position may be easier for patients with disabilities to transfer onto and off of than examination tables that are horizontal only.
• Sub-Committee Recommendation:
No revision.
M301.4 Lift Compatibility
M01.4.1 Clearance in Base
Proposed requirement:
The base of the equipment shall provide a clearance 44 inches (1120 mm) wide minimum, 6 inches (150 mm) high minimum measured from the floor, and 36 inches (915 mm) deep minimum measured from the edge of the examination surface. Where the width of the examination surface is less than 36 inches (915 mm), the clearance depth shall extend the full width of the equipment. Equipment components are permitted to be located within 8 inches (205 mm) maximum of the centerline of the clearance width.
• Sub-Committee Recommendation:
The base of the equipment shall provide a clearance 39 inches (1000 mm) wide minimum, 6 inches (150 mm) high minimum measured from the floor, and 36 inches (915 mm) deep minimum measured from the edge of the examination surface. Where the width of the examination surface is less than 36 inches (915 mm), the clearance depth shall extend the full width of the equipment. Equipment components are permitted to be located within 8 inches (205 mm) maximum of the centerline of the clearance width.
Rationale: Harmonization with IEC 60601-2-52 "Particular requirements for the basic safety and essential performance of medical beds" Change clearance requirement to:
39 inches (1000 mm) from 44 inches (1120 mm). This is the current International standard for clearance. Recommend that this be harmonious with this standard.
For stretchers with a shorter wheelbase than “standard” size stretcher bases, the clearance for a lift may include a lift window that is a combination of “Clearance in Base” and “Clearance Around Base”. With a short wheelbase, one leg of a floor based mobile patient lift will be placed through the base and the second leg of the lift will be placed around the base. The size of the window should still apply as written, these comments are placed here to identify a difference in how product configuration should not affect the clause.
IEC 60601-2-52 Particular requirements for the basic safety and essential performance of medical beds provides a similar requirement for lift clearance in the base. The dimensions there are 150mm x 1000mm. Recommendation here is to shrink the 1120mm dimension to 1000mm (39”) dimension to harmonize with the international standard.
M302 Diagnostic Equipment Used by patients in Seated Position
• Sub-Committee Recommendation
May be seen as applying to some versions of “Stretcher Chairs” used in Emergency Departments. These include products produced by Stryker, Hausted, Transmotion, etc.
Recommendation is that this section does not include “non-clinical” products (general chairs and/or recliners) used to support patients in a sitting position in “Fast Track”, “Urgent Care” or Triage settings.
It is important to create a distinction in the definition of these products so as to ensure that clinical products such as Examination Chairs and Stretcher Chairs fall under the extent of the rule, but that General Chair Seating or Recliners do not fall under the extent of the rule. Discussion regarding if stretchers fall under this criteria. Stretchers have a head & back support that keep the patient in a semi-reclined position, however the patients legs are not in a dependent position such as that when sitting in a chair. Stretchers are accessed when the stretcher is in a flattened position. Recommend that stretchers not be included in Diagnostic Equipment used by patients in a seated position.
M302.2 Transfer Surface
M302.2.3 Transfer Sides
Proposed requirement:
The transfer surface shall be located to provide options to transfer from a mobility device onto one short side (depth) and one long side (width) of the surface.
• Sub-Committee Recommendation: No revision as a stretcher chair should be the equivalent of a mobile examination chair.
M305.2 Transfer Supports
M305.2.2 Transfer Supports Structural Strength
Proposed requirement:
Transfer supports and their connections shall be capable of resisting vertical and horizontal forces of 250 pounds (1,112 N) applied at all points on the transfer support.
• Sub-Committee Recommendation:
Transfer supports and their connections shall be capable of resisting vertical and horizontal forces of 250 pounds (1,112 N) applied at the worst case accessible location without creating an unacceptable risk.
Rationale: Manufacturer shall determine the worst case accessible location(s) and test at these locations.
Acceptance criteria must be defined. It is understood that the Transfer Support will deform to some degree under these loads. This clause should limit elastic deformation and prohibit permanent deformation or breakage.
Possible language as modified from IEC 60601-2-52: “Transfer Supports shall be designed to withstand the forces applied during reasonably foreseeable use without creating an unacceptable RISK.”
As manufactures of medical equipment, ISO 14971 “Application of risk management to medical devices” is a commonly adhered to standard. As a result, product development normally includes the application of risk management practices and will consequently define “unacceptable risk” as it applies here.
M305.2.3 Fittings
Proposed requirement:
Transfer supports shall not rotate within their fittings
• Sub-Committee Recommendation:
Transfer supports shall not rotate within their fittings, when in the use or latched position, to the point of creating an unacceptable risk when subjected to the load conditions as prescribed in M305.2.2.
Rationale: It is understood that transfer supports will be of varying configuration and designs. Some may be removable, some foldable or articulating. In these cases, it may be advantageous to allow them to rotate, translate, fold, etc. but they should not do so at loads below those prescribed in M305.2.2.
References
IEC 60601-2-52 "Particular requirements for the basic safety and essential performance of medical beds"
ANSI/AAMI HE 75, section 16.4.7 (h.
Medical Diagnostic Equipment Accessibility Standards
Sub-Committee Recommendations
Weight Scales - Final Report
May 31, 2013
The Subcommittee’s technical recommendations for the weight scales have taken into consideration the following technical criteria proposed in Chapter 3, part 1195 to Title 36 of the Code of Federal Regulations. Specifically, section M303, patient positions equipment designed to support seated in a wheelchair.
Abbreviated Chart from Proposed Accessibility Standards for Medical Diagnostics Equipment Appendix to part 1195, Section M303.
| Patient Positions Equipment Designed to Support | Equipment Features Addressed in Technical Criteria | Types Of Equipment To Which Technical Criteria Applies |
| Seated in a wheelchair (M303) | Wheelchair space, including orientation, width, depth, knee and toe clearance, and surface slope Changes in level at entry to wheelchair space, including ramps Components capable of examining body parts of patients seated in a wheelchair, including height of breast platforms |
Imaging equipment designed for wheelchair use Weight scales designed for wheelchair use |
| Both seated in a wheelchair (M303) and in a standing position (M304) | Standing supports | Weight scales designed for both patients using wheelchairs and those standing |
Equipment Evaluated: Wheelchair weight scales with single ramps, dual ramps, stand-on scales and stow-a-weigh scales.
Types of Scales
| Single Ramp Scale (Diagram provided by US Access Board) |
Dual Ramp Scale (Picture provided by Scale –Tronix) |
 |
 |
| Portable Scales (Picture provided by Scale -Tronix) |
Wall Mounted or “Stow-A-Weigh” (Picture provided by Scale – Tronix) |
 |
 |
Sub-Committee Summary of Scope for Recommendations
Though sometimes avoided, all patients should have their weight taken as it is a basic screening measurement. It is sometimes difficult to take the weight of patients who have mobility or stability issues or for those who use wheeled mobility devices. However, without an accurate and current weight measurement, chances of missing diagnosis or incorrectly prescribing medications increase.
The goal of the sub-committee is to encompass the broadest range of individuals with mobility disabilities including wheeled mobility device users in the following recommendations. All standard size manual wheelchairs, powered wheelchairs, small to mid-range scooters and most bariatric size, extra wide manual chairs can be accommodated as it relates to the platform size recommendations; however, some of the larger roadside scooter models, typically a 4-wheeled base scooter, may not be accommodate by this platform size.
Rationale for including scooters in equipment evaluation: Scooter use is increasing as aging ‘Baby Boomers’ acquire mobility disabilities, etc. In 1994-95, 0.21% of elderly (65+) adults used scooters (64,000 people), according to our analysis of the NHIS-D published in 2000 in "Mobility Device Use in the United States." In 2011, 2.3% of elderly population 65+adults use scooters (about 800,000 people), according to data from the National Health and Aging Trends Study. Trend is more than 10 times as many people in 16 years use scooters.
Compare that to wheelchair usage among the 65+ elderly: 2.9% (900,000 people) in 1994-95, and 6.1% (2.1 million) in 2011. Double the usage.
Percentage of Scooter Users:
(Chart from NHIS-D published in 2000 in “Mobility devices Use in the United Sates”)
| User 65+ | Scooter | Wheelchair |
| Year | % / People | % / People |
| 1994-95 1 | 0.21% (64,000) | 2.90% (900,000) |
| 2011 2 | 2.30% (800,000) | 6.10% (2.1 million) |
| 16 year growth | 10 times | Doubled |
1 NHIS-D published in 2000 in "Mobility Devise Use in the United States"
2 National Health and Aging Trands Study
It is also acknowledged that not all scooter users can easily transfer from their scooters; transfers for scooter users may be just as difficult as those who are wheelchair users. Having an accessible weigh scale that can accommodate a wide range of patient populations and mobility devices is optimal as it saves the patient from having to transfer just to have their weight taken.
Rationale for including alternative equipment for taking weight: The Sub-committee acknowledges alternative types of equipment that have integrated scales to be an effective means of taking a patients weight. Alternative equipment with integrated scales can eliminate the need for unnecessary patient transfers to a weight scale just to capture a patient’s weight. For example, if a patient transfers onto an examination table that has an integrated scale, the healthcare provider can also capture the patient’s weight once the patient has made this single transfer. Such types of equipment with integrated scales may include; examination tables, stretchers, portable lifts and overhead ceiling lifts. The medical diagnostic equipment accessibility standards also apply to the alternative equipment for taking weight for each function if they include an integrated scale for the patient position it supports; prone, sitting, standing, seated in a wheelchair.
Proposed Recommendations and Sub-Committee Recommendations
M303.2.3 Wheelchair Spaces / Depth of Wheelchair Spaces for front or Rear Entry
• Sub-Committee Recommendation: The Sub-committee and Full Committee have decided not to act on the proposed recommendation by the Notice of Proposed Rulemaking (NPRM) and retain the proposed provision as is. Based on recent anthropometric data, NPRM proposes to increase minimum depth dimension from 48 to 58 inches – majority of members have no opinion or anticipation of a situation where requirement is needed.
Exceptions Considered for Wheelchair Spaces on Raised Platforms
M303.2.2 Proposed Exception: The exception to the minimum width in M303.2.2 would apply where ramped surfaces are provided on the opposite sides of the raised platform so that patients using wheelchairs can enter and exit the platform facing the same direction. The exception would permit the width of the wheelchair space between the edge protection to be reduced to 32 inches wide minimum at the platform level. This dimension is based on provisions in the 2004 ADA and ABA Accessibility Guidelines that allow accessible routes, which normally must be 36 inches wide minimum, to be 32 inches wide minimum for short distances such as at door openings. The exception would require a space 36 inches wide minimum to be provided outside the perimeter of the raised platform and above any edge protection so that patients using a manual wheelchair can extend their arms and elbows when they push on the wheel rims to maneuver onto and off of the platforms.
M303.2.3 Proposed Exception: The exception to the minimum depth in M303.2.3 for wheelchair spaces entered from the front or rear would permit a portion of the 48 inch minimum depth of the wheelchair space that accommodates the wheelchair footrests to extend beyond the raised platform and over any edge protection. For example, the wheelchair footrests would be allowed to extend beyond the depth of the raised platform and over any edge protection on wheelchair weight scales used by patients seated in a wheelchair.
• Sub-Committee Recommendation: Platform size shall be clear width of 32 inches minimum and clear length of 40 inches minimum.
(Figure 1 –Clear Platform showing the measurements of 32” wide x 40” inches length
Rationale: Clear platform size of 32 inchesminimum width x 40 inches minimum length accommodates non-powered and powered wheeled mobility devices including scooters; small and mid-size scooters. May not accommodate some larger roadside scooters with 4-wheeled based. This platform dimension is consistent with the recommendations of the IDeA Center Study recommendations for a minimum flat surface of 40 inches length for platforms accommodating wheeled mobility devices including scooters. This size platform allows for maneuverability on the platform for positioning and takes into consideration the operations of the wheelchair / scooter user to not always have the ability to stop precisely in the middle of the platform.
To have an accurate weight the wheelbase* 4 wheels and 3 wheels for some scooters must be on the platform (and make contact). The foot pedals, footrests, scooter deck and tip wheels can overhang or extend beyond the platform and still get an accurate weight.
*The IDeA Center Study defines wheelchair wheelbase as the distance from the center of the primary drive wheel to the center of the caster measured parallel to the floor.
*The IDeA Center Study defines scooter wheelbase as the distance from the center of the primary drive wheels to the center of the back or front wheels measured parallel to the floor.
*The IDeA Center Study findings indicate that a 36 inch length accommodates standard manual and motorized wheelchairs and most, if not all, bariatric size- extra wide manual chairs
IDeA Center Study at the University of Buffalo, New York): Summary analysis of wheelbase measurements on wheeled mobility devices
IDeA Center (Buffalo Study) Recommendations for Platform Length:
• a minimum flat surface of 40 in. length for platforms accommodating wheeled mobility devices including scooters
• a minimum flat surface of 33 in. length for platforms accommodating wheeled mobility devices excluding scooters
Ramps M303:3.3, 3.3.1, 3.3.2
M303.3 Proposed Provision: Changes in Level at Entry to Wheelchair Spaces includes technical criteria for changes in level at the entry to a wheelchair space as may occur at wheelchair weight scales with raised platforms. The technical criteria are consistent with the 2004 ADA and ABA Accessibility Guidelines. Level changes up to ¼ inch high are permitted to be vertical. Level changes between ¼ inch high and ½ inch high would be required to be beveled with a slope not steeper than 1:2. Level changes greater than ½ inch high would be required to be ramped. Ramp runs would be required to have a running slope not steeper than 1:12 and a cross slope not steeper than 1:48. The clear width of ramp runs would be required to be 36 inches minimum. Ramps with drop offs ½ inch or greater would be required to provide edge protection 2 inches high minimum on each side to prevent users from inadvertently travelling off the sides of the ramped surface.
• Sub-Committee Recommendation: Steepest Slope permitted to be:
• Rise 0 to 1 ½ inches – 1:2 Slope
• Rise >1 ½ to 2 ½ inches – 1:8* Slope
• Rise >2 ½ inches – 1:12 Slope
Rationale: Based on Weight Scales Subcommittee recommendation with full Committee’s input and partly the 2004 ADA and ABA Accessibility Guidelines. *The rise of >1 ½ to 2 ½ inches - 1:8 slope is from section 405.2 Maximum Ramp Slope and Rise for Existing Sites, Buildings, and Facilities - [A steeper ramp for a limited rise is allowable].
A ramp, using a 1:2 slope, will allow for short rise ramps found on stow-a-weigh scales. Space constraints are of consideration in this recommendation, as medical equipment and adequate space in the acute care or in the medical office setting are often competing. Scales that can be wall mounted or that are portable would facilitate where there are space constraints. Patients are always supervised and assisted by a healthcare provider when taking weight.
Current weight cell load technology that exists, allows for a low platform profile between ¾” to 1½”. As the height of the platform decreases, the length of the ramp can also decrease. The trend for the industry is towards lower weight cell technology development; however, this existing low weight cell profile may represent the limits of technology.
• Sub-Committee Recommendation: Edge Protection
o Single ramp scales: 2 inch edge protection absolute is required opposite the entry ramp on the platform as a safety feature to prevent wheeled mobility device drivers from over-shooting the platform as not all devices stop immediately when braking.
o A 2 inch maximum height is recommended so the edge protection does not obstruct the footrest from hanging over.
o Edge protection is required for either side of the platform (front edge and rear edge) and ramp. This is an additional safety recommendation.
Single and dual ramp scales: 2 inches minimum required on both sides (front edge and rear edge) of platform
Single and dual ramp scales: 2 inches minimum required on both sides of entry ramps
Not required where platform is ≤ 1½ inches
Rationale: Edge protection provides additional safety features so user of wheeled mobility devices do not inadvertently drivel off ramp and/or platform.
Single Ramp Scale with 2” Edge Protection
Opposite the ramp entry and on both sides of the platform and ramp
Figure 2: Picture of single ramp scale showing the 2” edge protection at the opposite side of the platform and showing the 2” minimum edge protection on both sides of the ramp and platform
Dual Ramp Scale with 2” Edge Protection
On both sides of the platform and ramp
Figure 3: Picture of a dual ramp scale showing the edge protection on both sides of the platform and ramp
Standing Supports (M304.3 and M305.3)
M304.3 Proposed Provision: Would require standing supports to be provided on each side of the standing surface on diagnostic equipment used by patients in a standing position. M305.3 would require the standing supports to provide continuous support throughout the use of the diagnostic equipment and to not rotate within their fittings.
M305.3 Proposed Provision: Also provides technical criteria for standing supports in horizontal and vertical positions. Standing supports can be provided in a horizontal position, vertical position, or a combination of horizontal and vertical positions, as long as the minimum length of gripping surface is provided for the support position used on each side of the standing surface. Standing supports that adjust from horizontal to vertical positions and at angles in between, such as a bar that folds up and locks into multiple positions, can be used. These kinds of adjustable supports are not required but would accommodate a broad range of patients with disabilities, particularly where a patient needs to assume multiple body positions for a diagnostic procedure or needs to step up onto a surface and then maintain balance afterwards.
• Sub-Committee Recommendation: Standing Supports – Location
• (scales serving both wheelchair and standing users).
o 1 side of platform for dual ramp entry of platform
o 2 sides of platform for single ramp entry with 34” minimum width between standing supports
o The standing supports need to be tied in to the weighing platform for weight accuracy.
Rationale: Standing supports required on one side of dual ramp has relevance for users being able to access either side for entry – user may only have one usable side due to condition such as a stroke, or right or left side paralysis or weakness etc.
Standing supports required on both sides for single ramp entry to assist user to gain access to weight platform.
A 34” minimum width between the supports allows for adequate elbow space to push wheels onto platform and does not infringe on the 32” platform.
Standing Supports (scales serving both wheelchair and standing users)
• Sub-Committee Recommendation: Standing Supports - Length
The length of the standing supports to be 32” minimum for single ramp scale and 40” minimum for dual ramp scale.
o Single Entry Ramp - On both sides of single entry platform for a length of 32 inches minimum starting at platform entry edge.
o Dual Entry Ramp – On one side of dual entry platform for a length of 40 inches minimum.
Rationale: Having standing supports for 80%, or 32 inches minimum length at the platform entry edge on the single entry ramp allows for enough coverage in length to assist the patient for stability to grab on both sides, to gain access to the platform for this one way entry and exit. Standing supports on the dual entry ramp, allows for the full length of the platform to have a standing support available on one side for the patient to provide stability when entering onto the platform and exiting off the platform - a user may select which side of the platform to enter given the limitation of having one strong side due to conditions such as stroke or right or left side paralysis or weakness etc.
Standing Supports - Single Ramp Scale
Figure 4: picture of standing supports on both sides of a single entry ramp scale showing the 80% coverage (or 32” length) starting at the platform edge and the 34” minimum space between supports
Standing supports – Dual Ramp Scale
Figure 5: picture of standing supports on one side of a dual ramp scale of the platform length of 40” minimum
References:
NHIS-D published in 2000 in "Mobility Device Use in the United States."
IDeA Center (Buffalo Study) wheelbase measurements on wheeled mobility devices
IDeA Center, University at Buffalo
Memorandum:
Date: 4/23/2013
From: Clive D’Souza and Edward Steinfeld, IDeA Center, University at Buffalo
To: Rex Pace, Technical Assistance Coordinator, U.S. Access Board
RE: Revised Knee Clearance Depth and Footrest Clearance dimensions for sizing of mammography equipment
Introduction
This memo provides a revised description and analyses examining knee clearance dimensions in mammography equipment (as requested in your email dated: 4/15/2013). The analyses pertain to:
1. Abdomen depth (AD)
2. Footrest clearance height and depth
1. Abdomen depth (AD)
Abdomen depth was analyzed as the overall clearance depth beneath the mammography machine, and measured from the anterior-most aspect of the abdomen to the anterior-most aspect of the occupied wheelchair (e.g., to the footrest or toe-tip). The data were analyzed using both, univariate and multivariate methods.
Univariate analysis
The univariate analysis examines AD alone and is appropriate when no other clearance dimension is constrained or bounded (e.g., knee clearance height, foot clearance height or depth). Table 1 and Figure 1 show the percentiles and maximum observed values for male and female wheelchair users. Within device type, female users had slightly less AD compared to male users. The 95th percentile value for female users of manual and power wheelchairs was approximately 30.5 in. and would accommodate about 95% of female users assuming no other clearance dimensions is restricted or bounded. Based on the current study sample, accommodating the entire user population would require a clearance depth between 33 in. – 37 in.
Table 1: Percentile and maximum values for Abdomen Depth (in.) obtained from univariate analyses
| Percentile Value | |||||
| User Group | Median – 50th | 75th | 90th | 95th | Max |
| Female MWC users (n=130) | 23.2 | 26.4 | 29.2 | 30.6 | 33.0 |
| Male MWC users (n=147) | 24.8 | 28.3 | 31.3 | 32.9 | 37.7 |
| Female PWC users (n=89) | 21.6 | 24.8 | 28.1 | 30.4 | 36.7 |
| Male PWC users (n=100) | 24.6 | 27.7 | 31.2 | 32.0 | 34.0 |
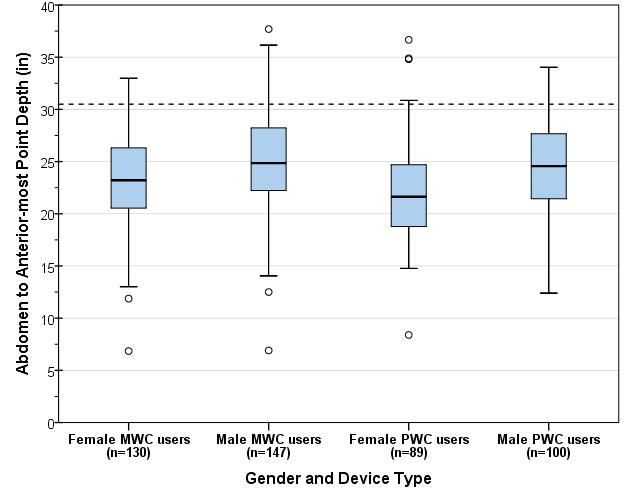
Figure 1: Boxplot showing the distribution for abdomen depth (inches) across wheelchair type and occupant gender. The dotted line (30.5 in) depicts the 95th percentile value for female wheelchair users, both manual and power.
Note on Multivariate Analysis
The above univariate analysis is only applicable in the case of one critical dimension (i.e., abdomen to anterior-most point depth) and all other knee clearance dimensions are unrestricted or unbounded. If more than one dimension is bounded (e.g., combinations of knee clearance height or depth, foot clearance height or depth, occupied width, etc.) then a multivariate analysis which involves simultaneous consideration of multiple dimensions is more appropriate for performing clearance assessments. As the number of dimensions in the multivariate analysis increases it is possible that the resulting space requirements are larger in comparison to the univariate case where each dimension is analyzed separately.
2. Analysis of footrest clearance height and depth
Any base support or flange for the mammography machine at floor level (inside the clear floor space width) would obstruct some wheelchair users from approaching the mammography machine. The purpose of this analysis was to determine suitable dimensions for the base flange depth and thickness at floor level to minimize obstructions in forward approach for such users.
Over 30% of manual wheelchair users in this sample did not use footrests. Detailed footrest measurements (i.e., foot rest height and corner points on the top surface) were collected from 158 manual wheelchair users and 108 power wheelchair users. Footrests were present and recorded on the right-side for 101 (64%) manual wheelchair users and 105 (97%) of power wheelchair users. Front castor and footrest measurements were available for 67 power wheelchair users (excluding front-wheel drives for this analysis, due to time constraints). Three dimensions were computed on the right-side for the sub-set of wheelchairs with footrests:
-
Abdomen-Castor edge depth: measured as the horizontal distance from the abdomen to the distal (forward-most) edge of the front castor measured at castor hub height
-
Abdomen-Footrest distal depth: measured as the horizontal distance from the abdomen to the distal (forward-most) edge of the footrest
-
Footrest height: measured as the height from the floor to the top surface of the footrest at the proximal (near) outside corner
Both depth dimensions are referenced to the abdomen assuming that the wheelchair occupant makes contact with the breast-platform at the abdomen. The range and median values measured on the right-side for these three dimensions are presented in table 2. Critical values are highlighted in yellow. For each dimension, the more conservative estimate across manual and power wheelchairs is recommended. Based on the data in Table 2 we recommend that:
-
The total knee clearance depth should preferably be no less than 30 in. to minimize contact with the footrest (i.e., 95th percentile value for Abdomen-Footrest Distal depth). The abdomen-anterior-most point depth presented in the previous section is a preferred dimension as the feet/toes often go past the footrest.
-
Any flange or base support should preferably be no deeper than the overall clearance depth (e.g., 32 in. or 30 in.) minus the 95th percentile value for abdomen-castor edge depth. For example, for power wheelchair users, recommended flange depth = 30 in. – 19.3 in. = 10.7 in.
-
Any flange or base support thickness should preferably be less than the 5th percentile value of footrest height for manual wheelchair users minus the thickness of the footrest (assuming 0.5 in.), i.e., approx. 1.7-0.5 = 1.2 in.
Table 2: Observed ranges and percentile values for Abdomen-Castor edge depth, Abdomen-Footrest edge depth, and Footrest Height, measured on the right-side for manual and power wheelchair users (Note: combined data for male and female). Critical values are highlighted in yellow.
Figures 2 and 3 provide scatter-plots showing the relationship between Abdomen-Castor edge depth (and castor hub height) and Abdomen-Footrest distal depth (at footrest height) referenced from the abdomen point measured on the right side for manual wheelchair users (fig. 2) and power wheelchair users (fig. 3) respectively.
Assuming a 30 in. knee clearance depth, sample profiles of flanges based on the footrest measurements for manual and power wheelchair users are also shown. Although the flange depth and thickness is limited (e.g., 1-1.5 in.) the data suggests that the flange thickness could be increased closer to the base of the machine e.g., a triangular profile.
Further, it can be assumed that individuals that did not have a footrest (over 30% of manual wheelchair users) have some lower extremity mobility and would be able to lift and place their feet on top of the flange/base.
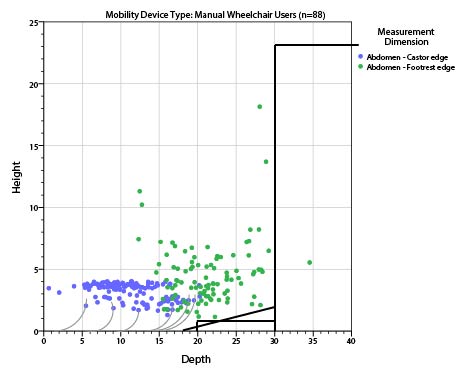
Figure 2: Scatter-plot showing observed values footrest height (on the vertical axis) and Castor-Footrest Depth (on the horizontal axis) measured on the right-side for manual wheelchair users referenced to the abdomen point. The grey curves show sample profiles of castor-wheels.
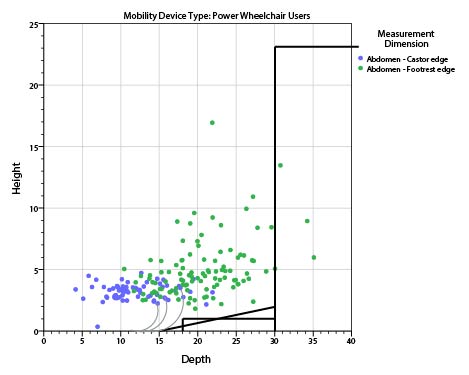
Figure 3: Scatter-plot showing observed values footrest height (on the vertical axis) and Castor-Footrest Depth (on the horizontal axis) measured on the right-side for power wheelchair users referenced to the abdomen point. The grey curves show sample profiles of castors-wheels.
Conclusions
Design criteria based on the univariate analysis of abdomen depth is sufficient if all other clearance dimensions are unbounded (i.e., unrestricted) and abdomen depth is the only relevant dimension. The 95th percentile value for female users of manual and power wheelchairs was approximately 30.5 in. and would provide adequate clearance depth such that approximately 95% of female users can approach the machine up to the abdomen.
Analysis of the footrest clearance height, abdomen-castor depth and abdomen-footrest depth suggests a flange of depth 10-10.7 in. and thickness 1-1.2 in. maximum. However, a graphical plot of the data suggests that the flange thickness could be increased closer to the base of the machine e.g., with a gradient or triangular profile.
IDeA Center, University at Buffalo
Memorandum:
Date: 10/23/2012
From: Clive D’Souza and Edward Steinfeld, IDeA Center, University at Buffalo
To: Rex Pace, Technical Assistance Coordinator, U.S. Access Board
RE: Analysis of Transfer Surface Dimensions Based on Wheeled Mobility User Anthropometry
Summary
The following report provides a brief analysis of four anthropometric dimensions which were considered relevant to the width and depth dimensions of a seating surface for wheeled mobility device users. The data are based on findings from the Anthropometry of Wheeled Mobility (AWM) Project, and comprises of detailed static measurements collected from 500 users of manual wheelchairs, power wheelchairs and scooters.
This analysis focuses only on the adequacy of the proposed transfer surface dimensions as a static seating surface – i.e., no measurements of transfers were recorded. The proposed dimensions for transfer surface 30in. wide and 15in. deep is estimated to be adequate as minimum criteria for a static seating surface. Our
research findings demonstrate that the minimum width of the surface could be as small as 28 in. and still accommodate 95% of our sample. It should be noted that Additional width may be required for accommodating users that are morbidly obese or use bariatric wheelchairs. Furthermore, additional space may be needed to accommodate the task of transferring, depending on the additional features provided, e.g. armrests or railings. User testing is recommended for arriving at more accurate transfer surface dimensions and design features to insure usability for a broad diversity of users, including individuals with mobility impairments.
Introduction
The proposed guidelines for medical diagnostic equipment recommends a minimum transfer surface 30 in. (760mm) wide and 15 in. (381mm) deep. At the request of the U.S. Access Board staff, a brief analysis was performed based on static anthropometry measurements previously collected to evaluate the adequacy of these transfer surface dimensions.
The Center for Inclusive Design and Environmental Access (IDeA) at the University at Buffalo conducted an anthropometric study of 500 individuals who use wheeled mobility devices (WMD)1. The research included the collection of demographic information and WMD characteristics, and the measurement of structural and functional anthropometry. Data collection included 3 dimensional measurements of approximately 90 points located on the body and device. Linear distances between pairs of measured points can be computed to provide dimensional estimates of body and mobility device sizes.
Four static body dimensions considered relevant to the width and depth dimensions of a seating surface were computed and analyzed to serve as a reference. These dimensions include: thigh breadth, shoulder breadth, buttock-popliteal length, and wheelchair seat depth. Dimension values are stratified by gender and mobility device type since significant differences in size and space requirements exist across these sub-groups. All dimensions are based on occupant static postures while seated in their own mobility device. As such, the analysis focuses only on the adequacy of the proposed transfer surface dimensions for a static seating surface. No measurements of transferring to or from the mobility device were recorded. Hence, the dimensions discussed in this report should only be considered as a starting point for accessible design of a seating surface. When determining space requirements for dynamic transfer tasks, there are additional space needs and functional abilities of the user population which must be considered. For example, individuals may need additional space to position their hands when conducting a side transfer. Issues of comfort and security need to be considered. Tasks that are performed on the table, like rolling over, need to be accommodated. Furthermore, the question of how to accommodate morbidly obese individuals still needs to be addressed.
The charts below were developed using a statistical analysis package with some built-in features. The reader will notice data points noted as “extreme values.” The statistical package analyses the data to identify “outliers,” or values that exceed expected values in data with a normal distribution. It then includes those values in the graphics as outside the range of maximum and minimum values in each distribution. The charts serve as a visual aid for understanding how the diversity or spread in measurement values within and between each of the six sub-groups of device users that were studied.
However, the “extreme values” are included in our assessment and recommendations on accommodating 95% of the sample, since these “extreme values” represent genuine cases of individuals (as opposed to errors in measurement) that need to be accommodated by design and hence should not be excluded from the analysis, and, the dimensions that we analyzed were not normal distributed due to a broad diversity in the observed measurements. Hence the percentiles were computed separately based on the observed data and not an implied normal distribution. Readers can assume that these “extreme values” represent unusually large or small dimensions for the population studied that might need special accommodation through design and/or some type of personal assistance.
1. Thigh Breadth
Thigh breadth was used to provide an estimate of the width of the seating surface. Thigh breadth for this analysis was computed as the larger of two dimensions, the horizontal distance between the lateral (outer) aspect of the hips, and the horizontal distance between the lateral aspects of the thighs, when seated in the wheelchair and not considering any wheelchair hardware. Figure 1 provides a box-plot showing the distribution for thigh breadth stratified by gender and mobility device type. It should be noted that the thigh breadth dimension for manual and power wheelchair users may be underestimated due to lateral posture support features (such as double-post armrest, wheelchair side guard). Scooters generally have single post armrests and hence the occupants’ thighs are laterally “unsupported” and tend to be wider reflecting a more natural posture. In wheelchairs, the armrests and side guards could compress overall breadth of the thighs to a certain extent.
Inadequate seat width can be more problematic for individuals that have broader hips and/or thighs. As such, the width dimension should be based on the higher end of the sample distribution to provide a sufficient base of support for as large a proportion of the user population as possible (e.g., 95th or 99th percentile). The 95th percentile and maximum values across the 6 sub-groups were within a range of 22in-28in, and the maximum values were within the 26in-30in. range. A minimum width of 30in would therefore ensure that all individuals of our sample would be accommodated in terms of the width of a static seating surface. If the minimum width were reduced to 28 in. only three individuals in our sample would not be accommodated.
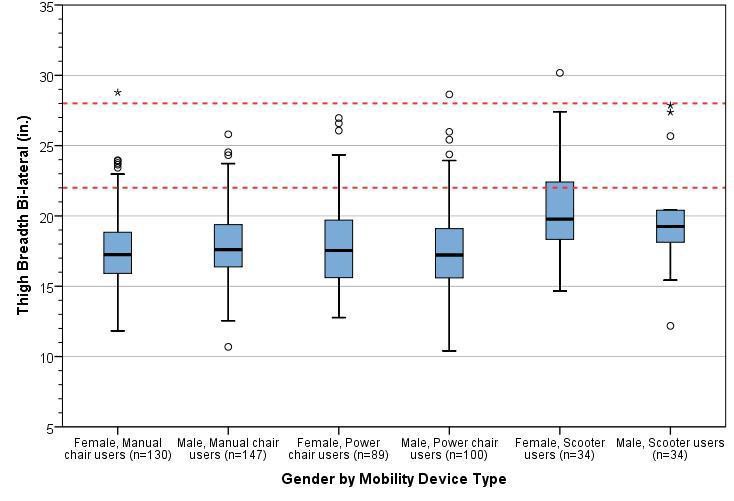
Figure 1: Box-plot showing the distribution for thigh breadth stratified by gender and mobility device type. The horizontal line splitting the box depicts the median, the box length represents the inter-quartile (25th – 75th percentile) range, and the whiskers represent the minimum and maximum values. Extreme values are shown as dots and asterisks. The red dotted lines depict the observed range of 95th percentile values across sub-groups.
2. Shoulder Breadth
Shoulder breadth tends to be slightly greater than hip or thigh breadth and hence was used as an estimate for the width of an examination table when in a reclined position. Shoulder breadth was computed as the horizontal distance between the lateral (outer) aspects of the deltoids. Figure 2 provides a box-plot showing the distribution for shoulder breadth stratified by gender and mobility device type.
Inadequate seat width can be more problematic for individuals that have broader shoulders and/or are obese. As such, the width dimension should be based on the higher end of the sample distribution to provide a sufficient base of support for as large a proportion of the user population as possible (e.g. 95th or 99th percentile). The 95th percentile values across the 6 sub-groups ranged from 23-27.5 in., and the maximum values ranged from 25-34 in. A minimum width of 30in. would ensure that all individuals of our sample would be accommodated in terms of static body width. However, space for tasks such as turning over to the side when reclining is not being considered.
A wider examination table (i.e., greater than 30in.) may be needed if accommodating individuals that are morbidly obese and/or use bariatric wheelchairs is also a priority. Our study sample is not representative in terms of the bariatric wheelchair user population – i.e., we had only one bariatric wheelchair user in the sample although we did have many people who were quite obese. The outlier values across thigh breadth and shoulder breadth might serve as a reference for determining the needs of morbidly obese individuals who use wheeled mobility devices (e.g., 34 in.).
Figure 2: Box-plot showing the distribution for shoulder breadth stratified by gender and mobility device type. The horizontal line splitting the box depicts the median, the box length represents the inter-quartile (25th – 75th percentile) range, and the whiskers represent the minimum and maximum values. Extreme values are shown as dots and asterisks. The red dotted lines depict the observed range of 95th percentile values across sub-groups.
3. Buttock-Popliteal Length
For this study, an estimate of buttock-popliteal length was calculated as the horizontal distance from the proximal (rear) edge of the wheelchair seat cushion to the popliteal fossa (i.e., crease at the back of the knee) of the right leg. The median buttock-popliteal length provides an upper bound for the depth of a seating surface. If seat depth is increased beyond this length, it becomes difficult to engage the backrest as well as exerting pressure to the back of the knees. Figure 3 provides a box-plot showing the distribution for shoulder breadth stratified by gender and mobility device type. The median (50th percentile) values for buttock-popliteal length across the 6 sub-groups ranged from 18.5-19.5 in. Seat depths should preferably not exceed these values (see next section for more information).
Figure 3: Box-plot showing the distribution for approximate buttock-popliteal length stratified by gender and mobility device type. The horizontal line splitting the box depicts the median, the box length represents the inter-quartile (25th – 75th percentile) range, and the whiskers represent the minimum and maximum values. Extreme values are shown as dots and asterisks. The red dotted lines depict the observed range of 95th percentile values across sub-groups.
4. Wheelchair seat depth
For this study, wheelchair seat depth was calculated as the horizontal distance from the proximal (i.e., towards the rear) edge to the distal (i.e., towards the forward) edge of the wheelchair seat cushion. Individual wheelchair seat depths are generally about 2 in. less than buttock-popliteal length in most case – this is a rough guideline and is quite similar to deciding seat depths for ambulatory individuals e.g., for office chairs. The median seat depth across different wheeled mobility devices would provide a reference value for the depth of other common seating surfaces. Figure 4 provides a box-plot showing the distribution for wheelchair seat depth stratified by gender and mobility device type.
The median (50th percentile) values for wheelchair seat depth across the 6 sub-groups ranged from 14-17 in. The proposed guidelines recommend a transfer surface depth of 15in. which is at about the middle of this range and may be appropriate.
Figure 4: Box-plot showing the distribution for approximate wheelchair seat depth stratified by gender and mobility device type. The horizontal line splitting the box depicts the median, the box length represents the inter-quartile (25th – 75th percentile) range, and the whiskers represent the minimum and maximum values. Extreme values are shown as dots and asterisks. The red dotted lines depict the observed range of 95th percentile values across sub-groups.
Conclusions
The proposed guidelines for medical diagnostic equipment currently recommend a minimum transfer surface
30 in. (760mm) wide and 15 in. (381mm) deep. Our findings support those dimensions but also suggest that
the width could be reduced to 28 in. Our analysis based on static anthropometry measurements. It focuses solely on the adequacy of these transfer surface dimensions for a static seating surface. No studies or measurement of transfers were recorded nor did we collect information on the space needs of other tasks on exam tables like repositioning the body for exams.
Based on an anthropometric analysis of four relevant dimensions, we have the following observations and recommendations:
-
The proposed minimum transfer surface width of 30 in. provides sufficient space as a static seating surface for almost all individuals in our sample of wheeled mobility users based on static measurements of hip and thigh breadth. This width could be reduced to 28 in. and still accommodate 95% of our sample.
-
A width of 30 in. would also accommodate the shoulder breadth of most individuals should this be required, e.g., when the table is in a reclined position. Reducing this width to 28 in. would still accommodate 95% of our sample.
-
Increasing the minimum width is recommended if accommodating individuals that are morbidly obese and/or use bariatric wheelchairs is also a priority. The maximum values recorded for thigh breadth and shoulder breadth serves as a reference value should accommodations for these users be necessary.
-
A depth of 15in. for a seating surface was found to be suitable based on median values of buttock-popliteal length and wheelchair seat depths.
-
These results reflect static anthropometry measurements and should be considered as a starting point for accessible design of a seating surface. User testing is recommended for arriving at more accurate transfer surface dimensions and design features to insure usability for a broad diversity of users and the full range of tasks that might take place on an exam table.
Notes:
1. Steinfeld, E., Paquet, V., D'Souza, C., Joseph, C., Maisel, J., 2010. Final Report: Anthropometry of Wheeled Mobility Project. Prepared for the U.S. Access Board. IDeA Center, Buffalo, NY.
IDeA Center, University at Buffalo
Date: 3/20/2013
From: Clive D’Souza and Edward Steinfeld, IDeA Center, University at Buffalo
To: Marsha Mazz, U.S. Access Board
RE: Summary analysis of wheelbase measurements on wheeled mobility devices
Wheelbase Definition
For the purpose of this analysis, wheelbase is defined as the distance from the center of the primary drive wheel to the center of the caster measured parallel to the floor. The larger of the dimensions measured on the right and left side of the mobility device were considered for the analysis.
The sample considered is the analysis of wheelbase is a sub-set comprising 242 the overall sample of 500 wheeled mobility users. Only one set of casters on mid-wheel drive wheelchairs were measured and so these devices are not excluded in the current analysis.
Table 1 shows the minimum, maximum and percentile values of the wheelbase dimension for manual wheelchairs, front- and rear-wheel drive power chairs, 3- and 4-wheel electric scooters.
Table 1: Range and percentile values for wheelbase (inches) by mobility device type
Introduction
The following memo is a brief analysis of the wheelbase dimension for wheeled mobility devices in the anthropometry of wheeled mobility database.
Observations:
-
Wheelbase dimensions for manual wheelchairs and rear-wheel drive power chairs were similar to each other but slightly less than the wheelbase for front wheel drive power chairs
-
Both 3- and 4- wheeled electric scooters had a larger wheelbase dimensions compared to manual and power wheelchairs. This sample had just three 4-wheeled scooters which limits detailed comparisons with 3-wheeled scooters
For platforms designed to accommodate wheeled mobility devices, additional space beyond the wheelbase should be provided. It would be very difficult for the occupant to have the powered device come to rest on a platform that is the exact length as its wheelbase or even a few inches more. Fine control (for example less than 1 in. increments) is difficult when operating powered wheelchair and scooter devices.
The wheelchair scale we used had a leveled surface of length 30 in. long with a bevel of 5 in. each at both ends (i.e., total length of 40 in.). Power wheelchair and scooter users often had trouble getting positioned exactly on the leveled portion of the platform requiring additional time and verbal assistance from an assistant/aide to get properly situated. Larger devices including power wheelchairs would often get positioned with two wheels on the bevel edge. We have observed that this causes an uneven weight distribution on the scale and could cause power wheelchairs to tip over when making short quick forward-reverse maneuvers in trying to get positioned on the platform. Including some aids to facilitate positioning would be a good idea also, for example, lines on either end with contrasting color and raised ridges at the ends of the level part of the platform.
Recommendations
Based on the data in Table 1 and the observations above, we recommend:
-
a minimum flat surface of 40 in. length for platforms accommodating wheeled mobility devices including scooters
-
a minimum flat surface of 33 in. length for platforms accommodating wheeled mobility devices excluding scooters
-
aids that can help wheelchair users mount the platform and position their chairs fully on the level surface.






User Comments/Questions
Add Comment/Question