Recommendations on Standards for the Design of Medical Diagnostic Equipment for Adults with Disabilities, Advisory Committee Final Report
Appendix A: Minority Reports
Boston Center for Independent Living, Inc.
Boston Center for Independent Living, Inc.
60 Temple Place, 5th Floor, Boston, MA 02111-1324
617 338-6665 (Voice) 617 338-6662 (TTY) 617 338-6661 (Fax)
866 338-8085 (Toll Free)
info@bostoncil.org This email address is being protected from spambots. You need JavaScript enabled to view it. (e-mail) http://www.bostoncil.org/ (website)
September 27, 2013
Mr. Rex Pace
Ms. Earlene Sesker
U.S. Access Board
pace@Access-Board.gov This email address is being protected from spambots. You need JavaScript enabled to view it.
sesker@Access-Board.gov This email address is being protected from spambots. You need JavaScript enabled to view it.
Re: Comments on Exam Tables and Chairs Subcommittee Report
Dear Mr. Pace, Ms. Sesker, and Members of the U.S. Access Board:
The following comments are provided regarding the Access Board’s Exam Tables and Chairs Subcommittee Report.
The Boston Center for Independent Living (BCIL) was founded in 1974 as the nation’s second independent living center, and annually provides services to over 4,000 people with disabilities in Greater Boston. It is a civil rights organization led by people with disabilities, advocating to eliminate discrimination, isolation and segregation by providing advocacy, information and referral, peer support, skills training and personal care attendant (PCA) services in order to enhance the independence of people with disabilities. BCIL specifically advocates for improved access to public transportation, employment, housing, government services, public accommodations, and health care. BCIL maintains an active membership, numbering over 500 people with disabilities.
As a member-led organization, with scores who have faced repeated discrimination in obtaining health care, BCIL has prioritized efforts to support equal access to health care. We have reached binding agreements with Massachusetts General Hospital and Brigham’s and Women’s Hospital, with a comparable one pending with the Boston Medical Center, to improve access to care and services for people with disabilities and ensure compliance with the Americans with Disabilities Act. In this work we have connected with many persons with significant physical disabilities who have encountered major barriers to care and services in a medical setting, and these persons’ experiences underlay the advocacy efforts that produced these agreements.
One of the most frequent concerns of these individuals was their inability to receive proper medical exams because they could not get on an examination table or because they were transferred to a table in an unsafe way. The consequence of examinations in wheelchairs was often second-rate care, undoubtedly resulting in poorer health outcomes in certain instances. In addition, even when transfer solutions were offered by medical practitioners, those responses often included dangerous and undignified lifting by personnel not trained to carry out the task. BCIL members have been unable to have gynecological exams, or have needed to be lifted by a husband onto tables, or were lifted, while undressed, by male orderlies or even untrained security guards. As a result, the barrier of inaccessible exam tables has produced another notable consequence: it discourages individuals from actually seeking needed care. A close associate has endured a year of chemotherapy and surgery for advanced cancer that may have been detected had she not avoided a precautionary exam in part because of the anticipated humiliation of having to explain—yet again— that as a paraplegic she could not stand to access her examination procedure.
Anything that inhibits proper transfers for people with disabilities, and not necessarily just those using wheelchairs, restricts care— which is both a profound civil rights violation and a major impediment to quality delivery of health care. A history of neglect by medical facilities regarding the simple aim of access to an exam table has harmed countless people with disabilities. A failure to mandate the best-possible access, something that would benefit people of short stature, including frail elders, little people, and children with disabilities, is not acceptable. The most expansive access possible is needed. For way too long people with disabilities have faced unequal access because of calculations made in the name of expediency and short-term cost savings.
The data provided to the Examination Tables and Chairs Subcommittee and articulated by medical practitioners in meetings and reported in the Examination Tables and Chairs Subcommittee report speaks to a low-height requirement of 17 inches for exam tables. BCIL strongly supports this, especially as we recall the late Frances Deloatch, a BCIL member who was involved in our advocacy work with Brigham and Women’s Hospital. For her, a two-inch lower exam table may have been critical. Frances had osteogensis imperfecta and was only three feet three inches tall. She once said that if her primary care physician had an exam table that lowered, she could transfer to it independently from her wheelchair. However, because of her stature, Frances had a child-sized wheelchair, and there is a very good chance that 19 inches would have been too high for her to self-transfer.
We cannot ignore individuals like Frances. A 17-inch height would provide greater accessibility and enhance the safety of transfers for small individuals. BCIL encourages you to develop the standard that will guarantee access to the greatest number of persons with disabilities possible.
Thank you for considering my comments on behalf of our members and for the board’s work to achieve equal access.
Sincerely,
Bill Henning
Bill Henning
Executive Director
The Brewer Company, LLC
Minority Report
The Brewer Company, LLC
01-Oct-2013
Final Report
Medical Diagnostic Equipment Accessibility Standards Advisory Committee
Section 5.1.3 Transfer Surface Low Height
Recommendation for 19” Low Height
The final report titled Advancing Equal Access to Diagnostic Services: Recommendations on Standards for the Design of Medical Diagnostic Equipment for Adults with Disabilities (Report) created by the Medical Diagnostic Equipment Accessibility Standards Advisory Committee (Committee) dated October 2, 2013 includes Section 6 which discusses the committee’s inability to reach consensus on the recommended lowest height for adjustable height transfer surfaces. As a potential resolution to this issue, Section 6.3 invites Committee members to submit their views regarding the low height specification in the form of a Minority Report.
As discussed in Section 6 of the Report, the Committee reached consensus on a high height specification of 25”. They also reached consensus on the need for continuous increments for the low to high height adjustment. Low height specifications of 17”, 18”, and 19”were considered but no consensus could be reached.
This Minority Report, submitted by Committee member Jack DeBraal on behalf of The Brewer Company, LLC, (Brewer) provides rationale for a 19” low height specification.
We request the Access Board to consider the following points in favor of the 19” low height:
1) The Subcommittee on Tables and Chairs convened by the U.S. Access Board’s Medical Diagnostic Equipment Technical Advisory Committee discussed the issue of transfer surface heights. Low height options of 17, 18 and 19 inches were discussed. After considering the available data along with the full Committee discussions, the Subcommittee concluded with a majority recommendation that examination tables and chairs shall have a low height of 19 inches.
2) The U.S. Access Board issued a Notice of Proposed Rule Making (NPRM) dated February 9, 2012 for accessible medical equipment. The proposed rule recommended that the height of the transfer surface during patient transfer shall be 17 inches minimum and 19 inches maximum measured from the floor to the top of the transfer surface. The Access Board based its proposal on provisions in the 2004 ADA and ABA Accessibility Guidelines for architectural features that involve transfers (e.g., toilet seats, shower seats, dressing benches). This is significant because:
a. According to these guidelines, a transfer surface with a fixed height of 19 inches meets the definition of accessibility.
b. Therefore, adjustable height examination tables with a low height of 19 inches would also meet the definition of accessibility.
Furthermore, toilet seats, shower seats, and dressing benches remain at their fixed height for both transfer and normal use. Adjustable height medical examination tables available today can be lowered to 19 inches to facilitate transfer, and can then be raised above 25 inches (the minimum high height recommended by the Committee) to facilitate the exam or procedure by the medical clinician.
3) The Committee attempted to determine the optimal accessible transfer surface height based on available data. In particular, the Advisory Committee spent a great deal of time discussing the Anthropometry of Wheeled Mobility (AWM) Project. This study measured the physical characteristics of people who use wheeled mobility devices and some of the characteristics of those devices. However, because the project only studied static positioning of users in their devices, it did not identify optimal transfer surface heights, and did not assess the ability of wheeled mobility device users to transfer independently onto an examination table or chair. In addition, the AWM Project measured the rear compressed seat height of the wheeled mobility device with the user seated in it. For transfer purposes, however, the most relevant height is the wheelchair front edge. The height of the front edge of the seat or cushion is important because the user will shift to the front of the seat to avoid having to lift over the side wheel to complete the transfer. Unfortunately, the AWM Project did not measure the front edge height. Consequently, the AWM Project data cannot be used to conclusively determine the transfer surface height required to accommodate independent wheelchair transfer.
4) Brewer has been manufacturing adjustable height examination tables since 2002. These tables were designed specifically for wheelchair accessibility by meeting the 19 inch height referenced in the ADA/ABA Accessibility Guidelines. Brewer is ISO 13485 certified. ISO requires a robust method for recording customer, end user, and clinician feedback. In the 11 years we have been selling adjustable height examination tables we do not have a single complaint on record regarding the accessibility of our 19” low height tables. There have been no requests for a lower height. In addition, market growth of adjustable height tables with 19 inch low heights (section 2.4.1 of the Report) provides further evidence that these tables are meeting the accessibility needs of patients requiring independent wheelchair transfer.
5) Brewer concurs with the cost versus benefit conclusion discussed in Section 8.4 of the Report. The benefits and effectiveness of any new accessibility standards should be quantified. As the cost associated with the implementation of new accessibility standards increases, the offsetting benefits should increase in equal proportion. It is certain that the addition of transfer supports and leg supports will add to the product cost. However, maintaining the current 19 inch low height will mitigate any additional costs that would be incurred with redesign of the lifting structure. The costs required to redesign existing adjustable height tables that already meet a 19 inch low height is difficult to rationalize without any tangible offsetting benefits. As section 8.4 states, research on the costs and benefits of improving accessibility requires further attention.
In conclusion, The Brewer Company, LLC as a manufacturer of adjustable height tables, will make a significant investment to comply with the new requirements for transfer surface size, transfer supports, and leg supports. These changes have been well justified in the Report and are supported by Brewer. However, there is no quantifiable data that justifies a revision to the currently acceptable low height range of 17 to 19 inches required by existing accessibility standards. An adjustable height table achieving a low height of 19 inches is considered accessible by these standards. There are no quantifiable benefits associated with a low height below 19 inches, although there would be a significant investment to redesign the lift structure and anticipated additional product cost.
Hologic, Inc.
Hologic, Inc.
36 Apple Ridge Road, Danbury, CT 06810 USA
Main: +1.203.207.4500 Fax: +1.203.207.4596
http://www.hologic.com/
MINORITY REPORT: CONSIDERATIONS FOR THE APPLICATION OF ACCESSIBILITY STANDARDS TO PRONE BREAST BIOPSY TABLES
John LaViola, GVP, Women’s Health Group, Product Development, Hologic, Inc.
Michelle Lustrino, Mechanical Engineer, Product Development, Hologic, Inc.
Prone breast biopsy tables were considered by the “Imaging Equipment with Transfer Surfaces” sub-committee and are described in Section 4.3.2 of the full committee report. As discussed in the report, prone breast biopsy tables are one of several equivalent clinical options used for specialized interventional breast biopsy procedures. Minimally, they require local anesthetics, and at times require some level of patient sedation. Section 4.3.3 of the full committee report states, “The Committee viewed imaging systems used for interventions and biopsies in patients who are typically sedated as outside the scope of the accessibility standards.” Although, in many cases prone biopsy procedures require only local anesthetics, prone breast biopsy tables should be exempt from the related transfer height and lift compatibility requirements under consideration by the committee’s standards and rules determination processes. Therefore, Hologic is submitting this report to support this exemption by describing the relevant clinical use cases and the practical application of the committee’s consideration of alternative lift compatibility requirements.
The process of detecting breast cancer begins with regular mammography screening exams. If an area of concern has been detected, then the patient may be asked to have more images of their breast taken in what is considered a diagnostic mammogram. It is only after these images are taken and a region of interest (e.g., calcifications or suspicious architectural distortion) has been detected that a patient will be required to go for a minimally invasive image-guided breast biopsy procedure. A breast biopsy is a minimally invasive surgical procedure used to sample tissue from a region of interest, and is the last of multiple steps required to diagnose breast cancer. While it is an important step in the diagnosis, it is the screening mammogram that is used to determine suspicious regions of interest and begin clinical work-up of these regions. It is critical that women have independent access to screening mammography equipment and that they can be examined with as much ease as possible, as independent access will impact their likelihood to get their regular mammograms. However, breast biopsy procedures are prescribed in response to a specific clinical concern with multiple healthcare and accessibility considerations jointly evaluated by the patient and the care provider. Accordingly, independent patient accessibility to breast biopsy procedures is not the overriding clinical concern and would not likely interfere with the patient’s access to health care.
Breast biopsies may be performed with the patient in a prone, seated, or decubitus position. A dedicated prone breast biopsy table is used only for biopsy procedures in the prone position. Equivalent care can be provided with biopsy accessories attached to upright mammography equipment with patients in seated or decubitus positions. It should be noted that for lesions that can be readily localized with ultrasound imaging, supine breast biopsies are typically performed. Additionally, some clinical situations may indicate an open surgical biopsy with the patient under general anesthesia.
It is critical that there be a method by which all patients can safely access medical diagnostic equipment. During a prone biopsy procedure (Figure 1), the patient lies in the prone position on a cushioned table, with her breast positioned beneath the patient support surface while the physician works underneath the table, out of sight of the patient. This configuration increases patient comfort and thereby is an advantage for the use of prone biopsy tables.
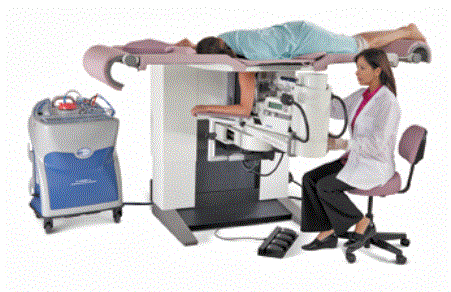
Figure 1. Example prone breast biopsy table. The patient is lying in the prone position on a cushioned table, with her breast positioned beneath the patient support surface while the physician works underneath the table.
Because the procedure takes place underneath the patient support surface, provisions are needed under the table for imaging and biopsy equipment and for a drive mechanism to move the table to a height that allows comfortable physician access, while also maintaining access to the patient from the front and back of the machine. As a result of these technical and clinical use constraints, the minimum possible table height in the table shown in Figure 1 is 37 inches. With the table surface at these heights, all patients are assisted in getting onto the table either with a step stool or with a supplemental patient transfer device (e.g., a stretcher). While future redesign may be able to decrease this height by a few inches, it would be cost prohibitive (and technically infeasible through practical means) to redesign the system to achieve any of the transfer heights described by Section M301 (even with the accessibility configurations discussed in Section 7 of the full Committee report).
This, however, does not mean that the prone biopsy table is inaccessible to patients in wheelchairs. While they may not be able to independently transfer onto the breast biopsy table, they can still access the table through transfer via an accessible stretcher (or other supplemental transfer device). In this case, the patient can independently transfer onto the stretcher, the stretcher will be raised to the height of the biopsy table, and the patient will transfer laterally onto the prone biopsy table. While this process will likely require the assistance of a technologist, the patient will be supported throughout the entire lateral transfer, unlike when they are transferring from their wheelchair to the stretcher, thus minimizing the safety concerns for the technologist and the patient. The technologist can utilize slip/slide sheets, boards, or other aids, to assist with lateral transfers.i Furthermore, if there are no obstructions to the transfer surface, the top of the stretcher and the top of the biopsy table can act as one continuous surface on which the patient positioning takes place, giving the patient ample space to use to turn over into the prone position. Once the patient is safely in position, the stretcher can be moved out of the way. Hologic understands the tremendous benefits of independent transfers, but due to the nature of this particular procedure, transfer via an accessible supplemental patient transfer device appropriately manages patient accessibility and use risks, and provides enhanced patient comfort during the procedure.
The above transfer procedure is an example of how using a supplemental patient transfer device provides a valuable method of assisted transfer as an alternative to the use of portable or overhead lifts. In the case of prone biopsy, this method is a safe, effective, and efficient way to transfer and position the patient. In cases such as these, where a stretcher-assisted transfer is used for its positioning and safety benefits, provisions for portable and overhead lifts are not required. If a patient requires a lift to transfer out of her wheelchair, she can use one for the initial transfer to the stretcher. In these cases especially, it will be important that the patient has ample space to move into the prone position, which will be made possible by the stretcher-based transfer method.
Seated position breast biopsies with an upright device provide the same efficacy and accuracy as prone biopsies,ii and therefore are equally effective in diagnostic work-up of the patient. If there are concerns of patient vasovagal response with the seated breast biopsy, they can likely be mitigated by decubitus positioning of the patient. Since these procedures take place using a screening mammography system, all patients will be able to achieve independent access to an upright biopsy and thus patients who are unable to access a prone biopsy table, even with the help of a supplemental patient transfer device, can still have a breast biopsy. However, it would be detrimental to the patients and to the healthcare system to rule prone biopsy systems inaccessible due to the technical constraints that minimize the ability for purely independent transfer.
All breast biopsy cases should be evaluated for their individual clinical and accessibility needs. When a breast biopsy is prescribed, the physician must work with the patient to ensure that the right biopsy procedure is chosen for them, taking into account any benefits or risks of that particular procedure. In order to maximize patient care, it is important that the prone biopsy table is included as an accessible option by this standards-setting process, provided that there is a plan in place to safely complete the assisted transfers at any site where this technology is used.
Hologic is fully committed to ensuring that our products are accessible to all patients and we appreciate the opportunity to work with the Access Board during this process. If you have any questions or need further information regarding the information presented here, please do not hesitate to let us know.
i. U.S. Department of Justice, Disability Rights Section. U.S. Department of Health and Human Services, Office for Civil Rights. “Americans with Disabilities Act: Access To Medical Care For Individuals With Mobility Disabilities.” Part 4: Accessible Medical Equipment. 15-16.
ii. Wunderbaldinger P, Wolf, G, Turetschek K, Helbich TH. “Comparison of Sitting Versus Prone Position for Stereotactic Large-Core Breast Biopsy in Surgically Proven Lesions.” American Journal of Roentgenology. 2002. 178:5, 1221-1225.
Midmark Corporation
RECOMMENDATION OF ALIGNING TRANSFER SUPPORT WEIGHT REQUIREMENTS WITH BIFMA STANDARDS
SEPTEMBER 27, 2013
SUBMITTED BY:
Midmark Corporation (Bob Menke, committee member)
BACKGROUND
Section 5.4.11 of the Committee report makes the following recommendation:
5.4.11 Transfer Support Structural Strength Recommendations for M301 and M302
Description: Transfer supports and their connections must be capable of resisting sufficient vertical and horizontal forces to remain stable during use. The proposed provision addresses these aspects of structural strength.
NPRM Proposed Provision: M305.2.2 Structural Strength. Transfer supports and their connections shall be capable of resisting vertical and horizontal forces of 250 pounds (1,112 N) applied at all points on the transfer support.
The Committee recommends transfer supports and connections contain the strength to resist vertical and horizontal forces of 250 pounds at locations determined by the intended use of the equipment.
Rationale for the recommendation
The Committee recommends a proposal that harmonizes the transfer support requirements with other provisions for transfer supports. The proposed technical criteria originated from provisions for grab bars in the 2010 Standards. During discussion manufacturers stated that industry is required to test the most vulnerable spots on the transfer support. Industry must follow testing parameters found in other standards. IEC60601-2-52: “Transfer Supports shall be designed to withstand the forces applied during reasonably foreseeable use without creating an unacceptable RISK.” Transfer supports will deform to some degree under these loads, it must remain stable enough for use. This clause should limit elastic deformation and prohibit permanent deformation or breakage. Manufacturers of medical equipment must follow ISO14971 “Application of risk management to medical devices”, a commonly followed standard. Product development manufacturers must use risk management practices to avoid any unacceptable risk defined by the specific product designs.
RECOMMENDED CHANGE
Revise the Section 5.4.11 recommendation as follows:
The Committee recommends transfer supports and connections contain the strength to resist vertical forces of 250 pounds and horizontal forces of 125 pounds at locations determined by the intended use of the equipment.
Rational for the change:
1. ANSI/BIFMA X5.1-2011 is widely recognized and used in the furniture industry. It specifies loading as percentage of patient weight (50% vertical and 25% horizontal). So for example, an examination table rated to support a 500 pound patient would be required to hold a weight of 250 pounds vertically, and 125 pounds horizontally.
2. ANSI/AAMI ES60601-1:2005, which is the safety standard most commonly used by medical devices, requires a similar instability test in section 9.4.2.3:
ME EQUIPMENT having a mass of 25 kg or more, other than FIXED ME EQUIPMENT that is intended to be used on the floor, shall not overbalance due to pushing, leaning, resting, etc. […]
The ME EQUIPMENT is placed on a horizontal plane and a force equal to 25 % of its weight, but not more than 220 N, is applied in any direction, except a direction having an upward component. […]
If the ME EQUIPMENT overbalances, it constitutes a failure.
Note that 220 N is equivalent to 49.5 pounds of force, significantly lower than BIFMA requirements.
3. Midmark has marketed a chair arm accessory for its examination tables for over 10 years that meets a load rating of 250 pounds vertically and 125 pounds horizontally. One of the intended uses of this chair arm is to assist with patient transfer (see image below). This chair arm has proven to be robust and reliable.

4. A horizontal force of 250 pounds is unrealistic. This far exceeds the force that a person can generate in the horizontal direction under any realistic transfer scenario (see figure below). Also, because MDE is not typically anchored to the floor, it is likely that the MDE would slide across the floor before a 250 pound force could be applied.
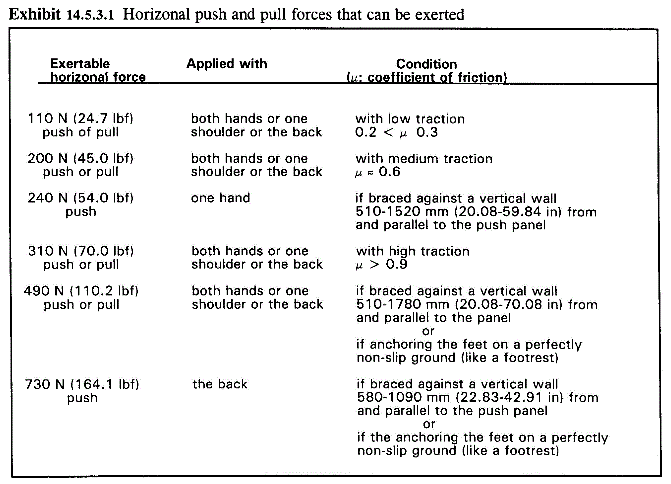
Source: FAA Human Factors Design Standard, Chapter 14
http://www.hf.faa.gov/docs/508/docs/hfdg/ch14.doc
National Network of ADA Centers
National Network of ADA Centers, Minority Report- Transfer Heights.
In Section 5.1, pages 66 and 69, justifications for a higher transfer height is made at 25” and chapter 6 (page 139) a more clear discloser is given for a high height number and the exclusion of a low height transfer number. The report says there was much disagreement for the low height transfer number and because of time frames, the high height number was never called to question.
Part of the confusion for not coming to a low height number was the fact that a high number was never clearly factored into the discussions, nor was the distance of movement of 8”
The proposed standards have a clear transfer height factored into their proposal of 17” to 19” and the advisory states that a seating height can move any distance up or down as long as the proposed transfer height was available. The proposed standards are simple and clear enough to meet all the concerns of manufacturers and people with disabilities. The minimum low and high height for compliance are stated and still allow for higher heights for motorized chairs, scooters, and people who ambulate using walkers, crutches or other type of mobility device
Much discussion was given to 17” and 18” heights being too low and manufactures wanted a 19” low height. If the proposed standards are accepted for transfer height the needs of manufacturers are met and compliance is achieved and the availability to move up and down to any other height is available after a transfer is made.
Since the proposed standards for transfer height are met. the National Network of ADA Centers proposes no change to M302.2.1,
M302.2.1 Height. The height of the transfer surface during patient transfer shall be 17 inches (430 mm) minimum and 19 inches (485 mm) maximum measured from the floor to the top of the transfer surface.
National Council on Independent Living
MDE Minority Paper for a 17 inch low Transfer Surface Height
Submitted to the U.S. Access Board By the National Council on Independent Living
September 27, 2013
In our opinion, this issue goes back to the beginning. Before we ever met as a Committee, the need for lowered transfer surface heights was identified with regard to exam tables and imaging equipment. In plain language, one need that started all of this was transfer surface – being able to get to the medical equipment for a diagnostic exam like everyone else. The surface height we had recommended historically was 17 to 19 inches above the floor, which has been around for years in existing Standards. If you have to pick an “absolute” low number, it only makes sense to pick 17 inches for the low height providing access to the greatest number of individuals with disabilities in order to access a standard of healthcare equal to all other people.
We should not be picking a new low height! 17 inches has always been the low height most commonly referenced in accessibility Guidelines and Standards. We already agreed to the high height in this application which is 25 inches. So this is just about the low number for accessibility - and that has already been established for decades as 17 inches!
Most of the information we received from other experts in the field, studies done on seat height of mobility devices (never mind all of the people who use other types of mobility devices or are short in stature), and our own experiences as people with disabilities pointed to having the lowest height available, at a minimum for initial access and egress from the equipment.
The manufacturers represented on the Committee consistently offered arguments based (whether they admit it or not) on the current equipment they have on the market, or what can be offered without much research and development or re-engineering (a.k.a. cost). Most manufacturers on the Committee had a 19 to 21 inch surface available currently, with at least one having a product at 18. Their argument has always been that providing the lowest transfer heights would be an extraordinary expense and burden on the business community (their consumer), not based on how it benefitted a patient with a disability. This effort was never supposed to be about the manufacturers or the doctors. It is the charge of this committee to answer questions and come up with recommendations for accessibility, based by some members on engineering and others by experience. NCIL’s 30-plus years of experience as advocates for people with disabilities dictates that we continue to strongly insist that the U.S. Access Board maintain the low accessible height at 17 inches above the floor in order for medical and diagnostic equipment to be accessed by the greatest number of people.
Dr. Ed Steinfeld’s study and testimony presented to the Committee recommended a height of 17 inches to provide the greatest level of access, and he himself stated 18 inches would be a compromise. The study showed that increasing the low end to 19 inch height excludes many users, specifically over 30% of female manual chair users and over 15% of male manual chair users. It wasn’t our charge to come up with a reasonable compromise. It was our charge to come up with what numbers provided the greatest level of accessibility. That number on the low end must be 17 inches or we are excluding a large segment of people who happen to use mobility devices from accessing an equal standard of care.
How the new standards are implemented – some over time, some right away, or not at all – that is to be determined by the authorities having jurisdiction for enforcement - like the Department of Justice, whose contributions to this committee process have been greatly appreciated. NCIL represents individuals with disabilities and IL organizations that are run by and for people with disabilities, as do many of the other advocacy organizations represented on the committee. We came to this committee because we were uniquely qualified to bring decades of experience making things accessible that were once not – based on the needs of consumers with disabilities. However with such technical equipment and engineering, it is not for us to provide all the technical answers to the roadblocks presented by some manufacturers represented on the Committee. The Subcommittees spent way too much time trying to solve engineering problems, when the point was to understand accessibility needs and meet them. If we drove all of our advancements over time based on what currently exists, we would still be in the stone age. We must make recommendations based on what will work best tomorrow to provide an equally high standard of care to more people with disabilities.
We should not recommend things based on what equipment currently exists. We must recommend things based on what people with disabilities need for equal treatment. The manufacturers are still trying to tell us what is best for us. We have challenged the medical model and tried to promote person-centered treatment for decades. We have NCIL members across the country who have not had the same standard of care as everyone else just because they use a mobility device – a wheelchair that may very well be 17 inches above the floor to the seat surface. They are who this rulemaking is for.
NCIL urges the Access Board to consider this and other minority reports calling for the 17 inch low transfer height, and to continue their rich history of providing accessibility that serves the greatest number of individuals with disabilities, and in this case provides much needed improvements in health care for Americans with disabilities including our Wounded Warriors.
Philips Healthcare
U.S. Access Board Federal
Medical Diagnostic Equipment
Accessibility Standards Advisory Committee
BALLOT ON COMMITTEE REPORT
This Ballot is due to the Access Board by October 11th, 2013
I, __Elisabeth George______,
(Print Name)
AGREE (with exceptions) that the recommendations included in the Advisory Committee Report dated September 13th, 2013 represent the consensus of the committee
With the following exceptions:
1) NPRM M303.2.4 Knee and Toe Clearance: Specifically regarding Mammography - Section 5.8.2 of the report.
• Refer to Pages 2 & 3 of this ballot.
2) NPRM M301.2.1 and M302.2.1: Table Height:– Section 5.1
• Refer to separate document from four medical diagnostic imaging equipment industry members.
DO NOT AGREE that the recommendations included in the Advisory Committee Report dated September 13th, 2013 represent the consensus of the committee
PLEASE NOTE: If you disagree with any of the recommendations included in the report, you are encouraged to submit a Minority Report describing your position on the specific recommendation with which you disagree.
Signed: ____________________________________________
(Signature required)
Representing: __Philips Healthcare ______
__3000 Minuteman Road Andover, MA 01810-1099____ Date: ______________
Please return this ballot by e-mail or fax to:
Rex Pace
U.S. Access Board
pace@access-board.gov
(202) 272-0080 (FAX)
Initials & Date: ----------------------------
EXCEPTION #2: NPRM M303.2.4 Knee and Toe Clearance
Specifically regarding Mammography - Section 5.8.2 of the report
The proposed recommendations focus on standard conventional and flat panel technology. However, there are also new emerging detector technologies, which are applied in state-of-the art mammography systems.
This mainly affects the breast support, because the breast support houses the detector, thus determining the dimensions of the breast support. This affects knee clearance.
Emerging technologies strive to improve mammography performance while increasing the safety of the devices by reducing women’s exposure to X-ray radiation.
Photon-counting technology has shown to deliver good mammography image quality while enhancing the safety of the procedure by applying a significantly lower dose of 40% or less, compared to other digital technologies.
The effectiveness of photon-counting technology in early cancer detection has been shown in European screening programs1,2. E.g. Baldelli et al.1 showed in the context of the Irish breast screening program, that despite recording the largest average compressed breast thickness (64.7 mm), the photon-counting system resulted in the lowest average examination mean glandular dose (1.86 mGy) compared with two other standard digital systems (3.03 and 2.91 mGy, respectively). The same radiologists’ performance at a dose reduced by 40% was also shown by Pisano et al.3
Photon counting systems imply a more voluminous breast support, as the detector itself is thicker4. However, by designing the lower detector housing smoothly sloped and curved, safe access is also given for women in wheel chairs.
References:
-
P. Baldelli et al: Comprehensive Dose Survey of Breast Screening in Ireland, Protection Dosimetry (2011), 145 (1), 52–60
-
B. Hemdal et al.: Average Glandular Dose in Mammography Screening using a Sectra MicroDose Mammography Unit, Protection Dosimetry (2005), 114 (3), 436-443.
-
E. Pisano et. al.: Comparison of Radiologist Performance with Photon-Counting Full-Field Digital Mammography to Conventional Full-Field Digital Mammography, Acad. Radiol. (2012): 19 (8) ,916-922.
-
M. Aslund et al.: Physical characterization of a scanning photon counting digital mammography system based on Si-strip detectors, Med Phys. (2007), 34(6):1918-25.
Image:
Philips MicroDose Mammography System
For additional pictures and brochures on device:
http://www.healthcare.philips.com/us_en/clinicalspecialities/WomensHealthCare/imaging/digital-mammography/
Medical Diagnostic Equipment Advisory Committee
MEDICAL DIAGNOSTIC EQUIPMENT ADVISORY COMMITTEE -
Minority Report Regarding: 17 Inch Low Transfer Surface Height
09.27.13
These organizations endorse this minority report.
- June Isaacson Kailes - Harris Family Center for Disability and Health Policy at Western University of Health Sciences
- Don Brandon -The ADA National Network
- Kat Taylor - Equal Rights Center
- Mark Derry - National Council on Independent Living
- Maureen Simmons - Paralyzed Veterans of America
- Kleo King - United Spinal Association
- Tamara James - Duke University and Health System
- Rochelle J. Mendonca - University of the Sciences in Philadelphia, Department of Occupational Therapy
A height range of 17 inches - 25 inches would accommodate a significant portion of people with disabilities. Data provided to the Committee and expert advice of medical practitioners led many to conclude that a lower height of 17 inches is essential to ensure safe transfers by patients with disabilities and thus, accomplishes accessibility for the greatest number of people.
Studies of Wheeled Mobility Devices and Transferring Abilities
Accessible medical equipment needs to facilitate safe transfers that accommodate the largest possible portion of people with disabilities, including people who use wheeled mobility devices. The safest and most easily accessible transfers are those with no or very little horizontal and vertical distance between the seat of the wheelchair and the transfer surface. Specifically, transferring to a higher surface applies greater exertion of the upper limbs.1
A study of wheeled mobility devices, including manual wheelchairs, power wheelchairs, and scooters examined the seat height of 495 users. The height was measured as the vertical distance from the floor to the lowest point of the seating surface of the mobility device, while the occupant was seated in the device. Thus, the surface of the mobility device was in a compressed state. The study noted that a range of 17 inches -25 inches accommodates the vast majority of wheeled mobility device users, while continuing to exclude 6% of manual wheelchair users whose devices are lower than 17 inches. Increasing the low end to 19” height excludes many users, specifically over 30% of female manual chair users and over 15% of male manual chair users.2
There is limited information on the ability of people with disabilities to transfer to a height different from the height of their wheeled mobility device. The Impact of Transfer Setup on the Performance of Independent Transfers: Final Report provides an analysis of the effect of height, horizontal gap, placement of armrests, and placement of grab bars on a person’s ability to transfer. The study noted that 86% of wheeled mobility device users could transfer to heights that were 2 inches above and below the height of their wheeled mobility device. However, this study was not representative of the diversity of wheeled mobility device users. Individuals were explicitly excluded from the study if they had significant upper extremity pain or injury that affects the ability to perform transfers, or had an active or recent history of pressure sores. Furthermore, the vast majority of subjects in the study were men.3 Numerous research studies as well as anecdotal reports from people with a variety of mobility disabilities (spinal cord injury, cerebral palsy, polio, traumatic brain injury, etc.) have detailed and reinforced that fact that people who live with disability experience a greater prevalence of and earlier onset of age related conditions such as arthritis, pain contractures, weakness, deconditioning, and shoulder injuries etc.4
1.The Impact of Transfer Setup on the Performance of Independent Transfers: Final Report. Presentation to US Access Board. Washington, DC. 2011
2. D’Souza, Clive and Edward Steinfeld, IDeA Center. Analysis of Seat Height for Wheeled Mobility Devices. 2011.
3. The Impact of Transfer Setup on the Performance of Independent Transfers: Final Report. Presentation to US Access Board. Washington, DC. 2011
4. Jensen, M.P., Molton, I.R., Groah, S.L., Campbell, M.L., Charlifue, S., Chiodo, A., Forchheimer, M., Krause, J.S., & Tate, D. (2011). Secondary Health Conditions in Individuals Aging with SCI: Terminology, Concepts, and Analytic Approaches. Spinal Cord, 50(5): 373-378.
Groah, S.L., Charlifue, S., Tate, D., Jensen, M.P., Molton, I.R., Forchheimer, M., Krause, J.S., Lammertse, D.P., & Campbell, M. (2012). Spinal Cord Injury and Aging: Challenges and Recommendations for Future Research. American Journal of Physical Medicine & Rehabilitation, 91(1): 80. doi: 10.1097/PHM.0b013e31821f70bc. Available from: http://journals.lww.com/ajpmr/Abstract/2012/01000/Spinal_Cord_Injury_and_Aging__Challenges_and.10.aspx. Accessed December 18, 2012.
Turk M. Secondary conditions and disability. In: Field MJ, Jette AM, Martin L (eds). Workshop on disability in America. A new look. Summary and background papers. Board on Health Sciences Policy, Institute of Medicine of the National Academies, The National Academies Press: Washington DC, 2006, pp. 185–193.
Kemp, B.J., & Mosqueda, L. (Eds.) (2004). Aging with a Disability: What the Clinician Needs to Know. Baltimore, MD: Johns Hopkins University Press.
Kailes, J. (2000). Health, Wellness and Aging with Disability, KAILES - Publications, http://www.jik.com/resource.html, jik@pacbell.net This email address is being protected from spambots. You need JavaScript enabled to view it. .
Kailes, J. (1995). "Midlife Cripdom: Getting Fewer Miles per Gallon?" The Disability Rag 16(4).
Kailes, J. (2001). Aging with Disability - Good News and Bad News. Western U-View. XX: 17.
Data Deficiencies
Advocates on the committee, representing the interests of people with disabilities, strongly stated that a 17” height is essential to accommodate the largest segment of people with disabilities. While general information can be found regarding average height of wheeled mobility devices, and people’s ability to transfer, data is not available regarding the needs of people of short stature and people with mobility disabilities who do not use wheeled mobility devices. The promulgation of standards for accessibility is required to take into account all people with disabilities, not just those who use wheeled mobility devices. In the absence of additional data, being inclusive versus exclusive is the most responsible approach in the development of standards.
Expert Advice of Medical Practitioners
Numerous practitioners commented on the importance of the availability of a lower transfer surface. Nüket J. Curran, PT, Director, Quality & Risk Management at UPMC Centers for Rehab Services, noted the importance of patient safety, staff safety, and ease of use. She stated that a 14 inch high transfer surface on tables and chairs would be ideal to facilitate the safest transfers, noting that 17 inches can be too high for some people. Ms. Lauren Snowdon, PT, DPT, Clinical Manager at the Kessler Institute for Rehabilitation, provided in depth analysis of the transferring abilities of wheeled mobility device users and the ideal height of transfer surfaces. Based on her experience that the general range of customized manual wheelchair height is 15.5 inches- 19.5 inches and that power wheelchairs range from 16.5”- 22” high, she stated that the option of a 17 inch high surface would be preferable to a 19 inch height. A 17 inch height allows for safer, easier transfers of patients whose wheelchair height is at the low end of those ranges. Other practitioners stated that the current height option of 18” was satisfactory and facilitated safe transfer for most patients. However, within this group, there was also a general consensus that a 17 inch height would provide greater accessibility, and enhance the safety of transfers for some patients. Given the importance of safe transfers and height ranges of wheeled mobility devices, a low height of 17 inches is considered by some to be a compromise solution.
The Limits of Utilizing a Cost-Benefit Analysis
Many advocates strongly cautioned against attempting a strict cost/benefit analysis when ample data is unavailable. To our knowledge there are no studies that provide a fully comprehensive analysis of the effects of the height of a transfer surface on people with various disabilities. Additionally, every time a patient with a disability is denied access to health care due to an inability to access equipment, the cost is often far more than traveling to a different health care facility. Delayed diagnosis and treatment translates into higher HEALTH CARE costs due to the need for more extensive and expensive treatment. Furthermore, the cost of the systemic denial of health care to these individuals can be life threatening.
There is additional risk to patients when medical practitioners attempt to manually transfer patients to diagnostic equipment. Risk of injuries due to being dropped, as well as skin shear and shoulder injuries can occur when the transfer surface cannot be adjusted low enough to accommodate a straight transfer for individual wheeled mobility devices of varying heights. The cost of medical practitioner injuries while performing these types of tasks has been widely documented and should also be considered.
While, there are always practical limitations on accommodating every individual, in the case of access to a service as essential as health care, advocates strongly urge the Access Board to develop standards that are not only practical to industry interests, but will guarantee access to the vast majority of people with disabilities.
1.The Impact of Transfer Setup on the Performance of Independent Transfers: Final Report. Presentation to US Access Board. Washington, DC. 2011
2. D’Souza, Clive and Edward Steinfeld, IDeA Center. Analysis of Seat Height for Wheeled Mobility Devices. 2011.
3. The Impact of Transfer Setup on the Performance of Independent Transfers: Final Report. Presentation to US Access Board. Washington, DC. 2011
4. Jensen, M.P., Molton, I.R., Groah, S.L., Campbell, M.L., Charlifue, S., Chiodo, A., Forchheimer, M., Krause, J.S., & Tate, D. (2011). Secondary Health Conditions in Individuals Aging with SCI: Terminology, Concepts, and Analytic Approaches. Spinal Cord, 50(5): 373-378.
Groah, S.L., Charlifue, S., Tate, D., Jensen, M.P., Molton, I.R., Forchheimer, M., Krause, J.S., Lammertse, D.P., & Campbell, M. (2012). Spinal Cord Injury and Aging: Challenges and Recommendations for Future Research. American Journal of Physical Medicine & Rehabilitation, 91(1): 80. doi: 10.1097/PHM.0b013e31821f70bc. Available from: http://journals.lww.com/ajpmr/Abstract/2012/01000/Spinal_Cord_Injury_and_Aging__Challenges_and.10.aspx. Accessed December 18, 2012.
Turk M. Secondary conditions and disability. In: Field MJ, Jette AM, Martin L (eds). Workshop on disability in America. A new look. Summary and background papers. Board on Health Sciences Policy, Institute of Medicine of the National Academies, The National Academies Press: Washington DC, 2006, pp. 185–193.
Kemp, B.J., & Mosqueda, L. (Eds.) (2004). Aging with a Disability: What the Clinician Needs to Know. Baltimore, MD: Johns Hopkins University Press.
Kailes, J. (2000). Health, Wellness and Aging with Disability, KAILES - Publications, http://www.jik.com/resource.html, jik@pacbell.net This email address is being protected from spambots. You need JavaScript enabled to view it. .
Kailes, J. (1995). "Midlife Cripdom: Getting Fewer Miles per Gallon?" The Disability Rag 16(4).
Kailes, J. (2001). Aging with Disability - Good News and Bad News. Western U-View. XX: 17.
MITA Advisory Committee Members
MINORITY REPORT ON TRANSFER SURFACE HEIGHT RECOMMENDATIONS OF THE U.S. ACCESS BOARD ADVISORY COMMITTEE
RE: NPRM: M301.2.1 AND M302.2.1 HEIGHT
I. Introduction
This Minority Report has been developed by the following medical diagnostic imaging equipment industry members of the Advisory Committee to the U.S. Access Board, including: GE Healthcare, Philips Healthcare, Siemens Healthcare, and Hologic, Inc.
The purpose of this Minority Report is to make a formal recommendation for a minimum transfer surface low height of 19 inches for diagnostic imaging medical equipment.
II. Recommendations of Diagnostic Imaging Equipment Manufacturers on Transfer Surface Low Height
The aforementioned medical diagnostic imaging equipment manufacturers support a 19 inch minimum transfer surface low height for the following reasons:
1. A standard should set a minimum transfer surface low height requirement which is reasonable and achievable given the technical and engineering resource constraints upon the equipment manufacturer.
Diagnostic imaging equipment is comprised of both the image detectors/scanner, such as an x-ray scanner, or nuclear medicine cameras, or imaging equipment using a bore, such as CT, PET and MRI, as well as the table supporting the patient. It should be recognized that the table, which supports the patient who is undergoing the diagnostic imaging procedure, is an integral part of
that equipment, and its precise alignment with the imaging scanner/bore itself is essential to achieving diagnostic quality images.
It is important to recognize, given the integrated nature of the table to the system and its imaging performance, that a change of even a few inches in minimum transfer surface low height constitutes a significant engineering change to the device. Any such change must ensure there are no adverse effects to image quality, system performance, and patient safety. Complete scanner
re-testing and re-certification under our formal FDA quality system and design controls are needed to verify overall system performance and safety.
Moreover, the most significant of these design changes can result in cascading alterations to the scanner, potentially leading to unacceptable heating in the case of MR, impacts on image signal/quality, and changes in dose levels to ensure the same, effective, high quality images and increased examination times, that is, additional workflow steps. It should be noted that future design projects are in the works many years before they become commercially available.
2. There was no compelling evidence presented of significant access improvement for diagnostic imaging devices at 17 inches that would warrant the additional efforts, timing and resources.
During the meetings of the Advisory Committee, there was no compelling evidence presented in support of a minimum transfer low height of 17 inches as the new standard for access. The original Notice of Proposed Rulemaking (NPRM) proposal was for a minimum height in the range of 17 to 19 inches. This would have allowed a table that lowers to a 19 inch minimum height to meet the standard. There did not appear to be sufficient, solid evidence presented that 19 inches minimum height was not adequate, and that 17 inches was necessitated to provide access. It should also be recognized, that due to the precision work which is required in the design of diagnostic imaging equipment, and the necessity of compliance with regulatory requirements, with each inch of decrease in minimum height from 19 inches, the time and costs which are required for equipment re-design go up exponentially.
However, the Committee did agree that an additional requirement, that of adjustability up to 25 inches, was critical to ensure access, given the variety of mobility device seating heights. We believe the addition of the adjustability requirement delivers by far a larger increase in access than having a single minimum height of 17 inches. As a standard , we believe the increased access goal is well met by having a 19 inch minimum height and transfer height adjustability. Having an additional extension to the NPRM to require a fixed minimum of 17 inches would provide only a marginal increase in the population served (see Item 3 below for further details).
3. While it is ideal for transfer surfaces to be at the same height as the seat heights of mobility devices, there is evidence that non-level independent transfers are also feasible.
The original Notice of Proposed Rulemaking (NPRM) Preamble explains the motivation for a minimum transfer height of 17 inches with the following:
“Transfer surfaces that are adjustable to the same heights as the seat heights of mobility devices reduce the effort needed to transfer since patients do not have to lift their body weight to make up the difference between the two surfaces, in one direction or the other.” 1
The NPRM then goes on to discuss wheelchair seat heights, with the 5th percentile lowest seat height being 17.3 inches, according to the IDeA study2.
While it is ideal for transfer surfaces to be at the same height as the seat heights of mobility devices, there is evidence that independent transfers are also feasible with a seat height discrepancy of up to two inches. As is discussed in Section 3.3.1 of the full Committee report, the Committee considered the findings from the University of Pittsburgh Human Engineering Research Laboratories (HERL) study that looked at transfers, and how different conditions affect the abilities of wheeled mobility devices (WMD) users to transfer from their WMD to another surface3. One of the goals of the study was to “determine acceptable ranges for non-level transfers (e.g. vertical height differences)”. A portion of the study’s results are shown in Table 1 below. It should be noted that the addition of a 3-inch gap next to the transfer surface (permitted by the Committee’s recommendation) may impact the percentages shown here, but information was not presented that directly correlated step height, gap, and ability to transfer. Further information would be needed to quantify this directly.
Table 1. A portion of the results presented in the HERL study to determine acceptable ranges for non-level transfers.
| Step Height (No obstructions or gaps between the transfer surface and WMD intentionally introduced) |
Percentage of Individuals Able To Complete The Transfer |
| 0” | 96% |
| 1” | 94% |
| 2” | 86% |
These findings suggest that those individuals with seat heights ≥18 inches high should be able to independently transfer to an adjustable-height transfer surface with a 19-inch minimum height, since the step in height will always be no more than one inch. For the small population of individuals with a seat height between 17.3 (5th percentile) and 18 inches, the maximum step height will be two inches. Based on this study, only about 14% of the individuals in this already very small population may need further assistance in transferring. In some instances, transfer or positioning supports may even be able to provide this assistance, thus maintaining independence of transfer. This study showed that adding a grab bar for patient support helped some wheelchair users to transfer at a height at which they could not transfer previously.
One of the Committee’s concerns regarding this particular study was that the study subjects did not necessarily reflect the general population of persons who use WMD. Of the people studied, 88 were men and 24 were women. A large number of subjects in the study were veterans who participated in organized sports-related events. The study did find, though, that the subjects’ daily activity levels apart from the time of those sports events did not differ from adult WMD users who live in the community. We do acknowledge the concern about the population studied and understand that the findings and percentages above may not provide strict guidance on how many people will be able to transfer given a non-level transfer situation. However, we believe that the insights from this study are very important to take into consideration when setting a minimum low height for transfer surfaces, since there are in fact many individuals who will be able to independently transfer with a 1 or 2-inch step height. While setting the minimum low height, we also believe it is important to specifically consider the individuals who will be impacted by this low height requirement. Many of the wheelchairs with low seat heights (especially in the 17-18inch range) are manual wheelchairs. Many manual wheelchair users may use more upper body strength in moving around for day-to-day activities than power wheelchair and scooter users do, and so a non-level transfer may not be as difficult for those individuals.
4. Diagnostic imaging devices require a trained technician present to aid all patients in accessing the table in the proper imaging position.
Diagnostic imaging devices operate with a high level of precision. It is important to recognize that proper patient positioning on the table for diagnostic imaging devices, whether they are x- ray, nuclear medicine, PET or MRI devices, is essential to achieving diagnostic quality images. In order to achieve diagnostic quality images, trained technologists are required to position all patients properly, regardless of the patient’s ability or disability to access the equipment.
1 Notice of Proposed Rulemaking (NPRM). Preamble, page 18.
2 IDeA Center Study at the University of Buffalo, New York: The Wheeled Mobility Anthropometry Project, Final
Report, page 49, Figure 3-5, reference to “All Device Types*”.
3 “The Impact of Transfer Setup on the Performance of Independent Transfers: Final Report”; Human Engineering
Research Laboratories, VA Pittsburgh Healthcare System, University of Pittsburgh.
III. Proposed alternative mechanisms to enhance patient access to diagnostic imaging equipment
Given the constraints on table redesign due to diagnostic needs, and physical and engineering limitations of many of the wide variety of imaging tables, there will be many types of imaging tables that will not be able to achieve even a 19 inch minimum if the table itself must be modified. For example, in a CT/PET system, the patient support surface is located on top of a transporter, which adds to the height of the transport surface.
DXA bone densitometry systems are currently at a fixed height of 25-28 inches because the imaging hardware is located beneath the patient and the precise position of the patient support surface relative to the x-ray equipment is critical to the clinical efficacy of the device. In the case of DXA systems, it may be most effective to specify a specific range of fixed heights that the table could be at to facilitate independent transfer, similar to the fixed height standards for everyday items like benches, bathing fixtures, and recreational structures.
For all other systems, the Committee was told that it needed to consider only modifications to the tables themselves. To the imaging industry this seems to be a rather arbitrary constraint, and one that is not reflective of the system nature of diagnostic imaging systems, their installations, nor an actual requirement in the law.
We believe that system accessibility configurations discussed in Section 7 of the Committee’s Report,
i.e., ancillary equipment that is not attached directly to the table, but rather that is part of the room layout, will need to play an essential role in enabling more imaging equipment to meet the scope of the proposed new standards. These alternative means can take many forms, and their specific designs would need to be worked out with appropriate stakeholders to maximize safety, patient handling, and of course, access.
Implementation of these alternatives, and others, will allow many more patients with disabilities to access the equipment, and can be implemented far more quickly and cost effectively than the significant time
and costs which would be required for equipment re-design. Many aspects of the system accessibility configuration concept would also be able to be applied to systems already in use, thereby increasing their accessibility.
The added benefits of implementation of these alternative means are the significant savings in healthcare costs, timelier implementation and broader system coverage which would be realized due to avoidance of costly, equipment re-design costs. Since our mutual goal is to enhance and improve access of patients to medical diagnostic equipment, we urge the Access Board to give serious consideration to these alternative means which will enable us to quickly accommodate many more patients with disabilities.
Conclusion
As diagnostic imaging equipment manufacturers, we are committed to achieving access of all patients to diagnostic imaging equipment. We stand ready to work with all stakeholders to accomplish this goal.
If you have any questions, or need further information, please do not hesitate to contact us. We would be pleased to further discuss these issues with you.
Sincerely,
John Jaeckle
(signed)
GE Healthcare
Elisabeth George
(signed)
Philips Healthcare
Hans Beinke
(signed)
Siemens Healthcare
Michelle Lustrino
(signed)
Hologic, Inc.
1 Notice of Proposed Rulemaking (NPRM). Preamble, page 18.
2 IDeA Center Study at the University of Buffalo, New York: The Wheeled Mobility Anthropometry Project, Final
Report, page 49, Figure 3-5, reference to “All Device Types*”.
3 “The Impact of Transfer Setup on the Performance of Independent Transfers: Final Report”; Human Engineering
Research Laboratories, VA Pittsburgh Healthcare System, University of Pittsburgh.
Recommendation of a 19" Lower Adjsutable Height as the Minimum Accessibility Standrds (Joint Report)
RECOMMENDATION OF 19-INCH LOWER ADJUSTABLE HEIGHT AS THE MINIMUM ACCESSIBILITY STANDARD
SEPTEMBER 27, 2013
SUBMITTED BY:
- The Brewer Company (Jack DeBraal, committee member)
- Hausmann Industries (David Hausmann, committee member)
- Medical Technology Industries, Inc. (Jeff Baker, committee member)
- Midmark Corporation (Bob Menke, committee member)
CONTENTS
- Background
- Summary of the 19-inch recommendation
- Current Situation: The Vast Majority of Examination and Procedures Tables Are 32 Inches High
- Implication of a 19-Inch minimum standard for the highest point in the lowest adjustable position
- Alignment of 19-inch Recommendation with Access Board Proposed Rulemaking
- A minimum highest point standard of 19 Inches is consistent with existing accessibility standards
- An Increasing number of Health Care Providers are Transitioning to Adjustable Height Tables
- Available data do not support departing from the currently accepted standard of 19-Inch transfer surface height
- Adoption of a 19 inch height minimizes costs to health care Providers
- Benefits and Costs: Overview
- Cost of Equipment
- Costs to Lower Minimum Table Height
- Scoping Scenarios: Range of Possibilities
- Benefits and Costs: Summary
- How to Measure Transfer Heights is Important
- Features
- WMD Positions For Transfer
- measurement Technique
- Seat Height Measurement Detail
- Conclusion
- Appendix A
- Appendix B
BACKGROUND
Section 4203 of the Patient Protection and Affordable Care Act (the “Affordable Care Act”) adds a new section to the Rehabilitation Act of 1973 requiring the U.S. Access Board to promulgate regulatory standards “setting forth the minimum technical criteria for medical diagnostic equipment used in (or in conjunction with) physician's offices, clinics, emergency rooms, hospitals, and other medical settings. The standards shall ensure that such equipment is accessible to, and usable by, individuals with accessibility needs, and shall allow independent entry to, use of, and exit from the equipment by such individuals to the maximum extent possible.” It also requires the U.S. Access Board to periodically review and, as appropriate, amend the standards.
Based on this, the Access Board is to establish minimum technical criteria for medical diagnostic equipment used in (or in conjunction with) physician's offices, clinics, emergency rooms, hospitals, and other medical settings.1 In response, the Access Board issued a notice of proposed rulemaking2 that, among other things, would specify a maximum lower adjustable height for the transfer surface. The intent of this minority report is to discuss the specific recommendation that the minimum standard for the highest point of the transfer surface in the lowest adjustable height should be 19 inches in sections M301.2.1 and M302.2.1.
NOTES
1. Patient Protection and Affordable Care Act, Pub. L. No. 111-148, §4203, 124 Stat. 119 (2010).
2. See Architectural and Transportation Barriers Compliance Board. Notice of Proposed Rulemaking: Medical Diagnostic Equipment Accessibility Standards, 77 Fed. Reg. 6916, February 9, 2012
SUMMARY OF THE 19-INCH RECOMMENDATION
The subcommittee on tables and chairs convened by the U.S. Access Board’s Medical Diagnostic Equipment Technical Advisory Committee discussed transfer surface height extensively. The subcommittee concluded with a majority recommendation that examination tables and chairs have a lower adjustable height of 19 inches or lower.3 A minority of the subcommittee did maintain that 17 inches was preferred, and when this 19 inch recommendation was discussed with the full Advisory Committee on May 7th, 2013, the full committee was unable to reach a consensus position or even a strong majority sentiment.
Although the subcommittee’s conversations were complex and far-reaching, the following points summarize the factors that were considered in the selection of the 19 inch height recommendation:
-
Equipment must be accessible to, and usable by, individuals with accessibility needs and will allow independent entry to, use of, and exit from the equipment by those individuals
-
Carefully balance the costs to hospitals, physicians, and other health care providers of replacing or modifying existing equipment together with manufacturer costs of redesigning equipment
-
Carefully balance the costs to hospitals, physicians, and other health care providers for providing alternative means of access (such as patient lifts, or staff providing lift assistance)
-
Minimizing costs to health care systems
-
Maximize rate of adoption of accessible equipment by health care providers and the benefits of that equipment to individuals with accessibility needs, particularly those who use wheeled mobility devices.
The minority report explains why a requirement for a 19 inch lower adjustable height for tables and chairs is the most appropriate standard for the initial implementation of section 4203 of the Patient Protection and Affordable Care Act.
NOTES
3. Note that height measurement, as defined by the tables and chairs subcommittee, represents the highest point of the transfer surface, inclusive of bolsters, when measuring to the top of uncompressed foam. See the “Measurements of Tables and Chairs” subcommittee report, dated April 5th, 2013.
CURRENT SITUATION: THE VAST MAJORITY OF EXAMINATION AND PROCEDURES TABLES ARE 32 INCHES HIGH
In the United States, approximately 82 percent4 of physicians, hospitals and other health care providers use examination and procedures tables with a 32-inch fixed height, as shown in Figure 1. Industry commonly refers to these tables as "box tables.” These tables provide an often-insurmountable barrier to health care for people with mobility disabilities. Since 2001, the number of adjustable-height tables has steadily increased, but continues to represent a minority of examination tables in the United States.
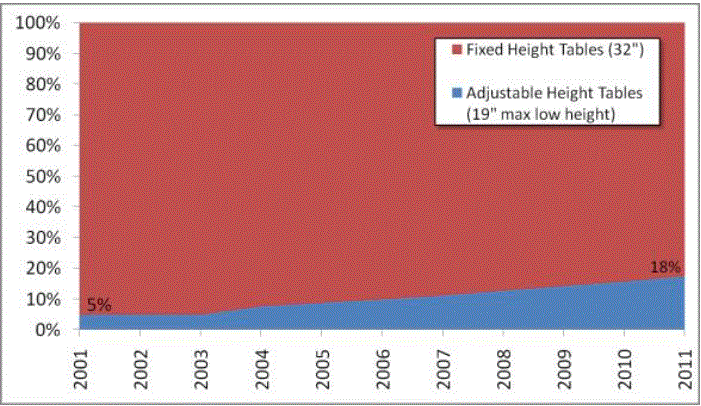
Figure 1: Percentage of fixed height versus adjustable height medical tables
(representing the U.S. install base of medical tables)
One of the primary objectives of the U.S. Access Board’s requirements should be to accelerate the growing trend of heath care providers to purchase adjustable height tables. In doing so, care should be taken to not render the progress that has already been made obsolete. For the reasons presented in this minority report, a 19 inch low adjustable height is best suited to achieving this objective. As illustrated in Figure 1 above, a standard lower maximum height that is less than 19” would re-classify the existing adjustable height tables available on the market today as inaccessible and penalize the physicians who have already made a good faith effort at accommodating their disabled patients.

Figure 2: Photograph illustrating the height difference between a fixed height and adjustable height medical table
NOTES
4. Medical table install base derived from U.S. medical distribution sales data, as provided by Global Healthcare Exchange (GHX), found at http://www.ghx.com/product-pages/solutions/supplier-solutions/sales-data-analytics.aspx
IMPLICATION OF A 19-INCH MINIMUM STANDARD FOR THE HIGHEST POINT IN THE LOWEST ADJUSTABLE POSITION
This minority report presents the factors that support a minimum standard of 19 inches as the highest point on the transfer surface in a table or chair’s lowest adjustable position. However, shorthand references to 19 inches as the minimum standard as used in this minority report should not be equated with a 19-inch transfer surface height, for two major reasons. First, as depicted in Figure 3, adjustable tables currently on the market generally feature contoured bolsters that provide greater security once an individual is seated or lying on the table or chair. A 19-inch standard means that any bolsters fit within the highest point standard, thereby making the front edge of the table/chair lower than the bolsters (by about ¾” based on currently marketed bolsters, or about 18 inches compressed at the transfer surface).
Second, as a minimum standard, establishing a 19-inch highest point standard does not mean that all newly manufactured tables and chairs will necessarily be fixed at a 19-inch height. Unlike fixed transfer surfaces such as toilets or non-adjustable tables, there is no reason to standardize at a single height based on broadest usability. In a marketplace of adjustable tables and chairs, increased range of adjustability will be advantageous to patients and caregivers alike. It is not unreasonable to expect that table and chair manufacturers will seek to compete by offering products with greater degrees of adjustability.
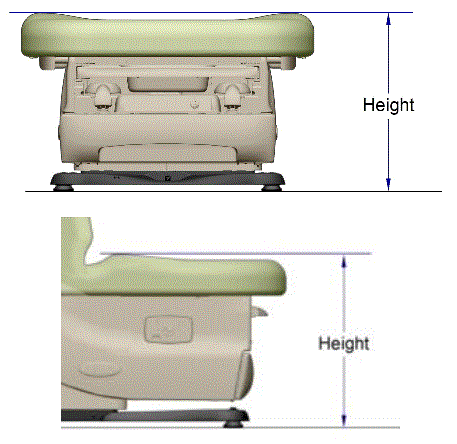
Figure 3. Illustration of measurement height at bolsters relative to lower transfer surface
ALIGNMENT OF 19-INCH RECOMMENDATION WITH ACCESS BOARD PROPOSED RULEMAKING
The subcommittee’s recommendation of a lower adjustable height of 19 inches maximum is consistent with the U.S. Access Board’s proposed rule and supported by public comments. In its proposed rule, the Access Board proposed that the “height of the transfer surface during patient transfer shall be 17 inches (430 mm) minimum and 19 inches (485 mm) maximum measured from the floor to the top of the transfer surface” for both examination tables and chairs.5 The Access Board based its proposal, “on provisions in the 2004 ADA and ABA Accessibility Guidelines for architectural features that involve transfers (e.g., toilet seats, shower seats, dressing benches).”6 In addition, the Access Board recommended, “Where patient support surfaces are contoured or upholstered for patient comfort or to support patient positioning during diagnostic procedures, the height of the transfer surface measured from the floor may vary across the transfer surface. The highest and lowest points of the transfer surface on such equipment would have to be within the specified dimensions.”7 The Access Board proposed that the measurement should be taken from the “floor to the top of the upholstery under static conditions, without compression or deflection in the transfer surface ….”8
The Access Board’s proposal also explained that it is considering a requirement in the final standards that the height of transfer surfaces be adjustable from 17 inches minimum to 25 inches maximum during patient transfer. In support of the alternative proposal, it cites ANSI/AAMI HE759 and the Wheeled Mobility Anthropometry Project.10 The results of this study recommended adjustable heights, with an increased maximum height above 19 inches, be provided in order to better accommodate users of powered wheelchairs and scooters. During the committee hearings, the manufacturers accepted that this would be appropriate and offered 19-inch to 25-inch adjustable height through powered tables.
The subcommittee’s recommendation appropriately balances the two proposed alternatives included in the Access Board’s proposed rule. Therefore, the Access Board should adopt the subcommittee’s recommendation provided for continuous adjustability of the height of the transfer surface between 19 and 25 inches.
NOTES
5. See M301.2.1 and M302.2.1.
6. See Architectural and Transportation Barriers Compliance Board. Notice of Proposed Rulemaking: Proposed Accessibility Standards for Medical Diagnostic Equipment. February 8, 2012.
7. Ibid.
8. Ibid.
9. Ibid, citing ANSI/AAMI HE 75, section 16.4.4. ANSI/AAMI HE75 recommends that the height of patient support surfaces "should be easy to adjust (ideally, powered) to suit the needs of health care professionals and patients." ANSI/AAMI HE75 further recommends that the height of patient support surfaces "should be adjustable to a position high enough to accommodate tall health care providers and the range of medical procedures that could occur . . .[and] to a position low enough [19 inches maximum] to allow for the comfort of providers who choose to work in a seated position, to enable patients to keep their feet on the floor while seated, and to accommodate patients who need to transfer laterally between the platform and a chair or wheelchair alongside."
10. See Analysis of Seat Heights for Wheeled Mobility Devices at: http://udeworld.com/analysis-of-seat-height-for-wheeled-mobility-devices. The seat heights ranged from 16.3 inches to 23.9 inches for manual wheelchair users; 16.2 inches to 28.9 inches for power wheelchair users; and 18.8 inches to 25.3 inches for scooter users. Seat heights for males were typically higher than for females. Thirty (30) percent of female manual wheelchair users and 6 percent of female power wheelchair users had seat heights equal to or less than 19 inches. All the male manual wheelchair users and 92 percent of the male power wheelchair users had seat heights equal to or less than 25 inches. Thus, transfer surfaces that are adjustable from 17 inches minimum to 25 inches maximum during patient transfer accommodate significantly more patients who use mobility devices.
A MINIMUM HIGHEST POINT STANDARD OF 19 INCHES IS CONSISTENT WITH EXISTING ACCESSIBILITY STANDARDS
Current accessibility standards and regulations generally consider a transfer surface with a fixed height between 17 inches and 19 inches accessible. Therefore, under these rules a transfer surface with a fixed height of 19 inches meets the definition of accessibility.11
Nineteen-inch water closet and toilet seats are accessible:
604.4 Height. The height of water closet seats shall be 17 inches (430 mm) minimum and 19 inches (485 mm) maximum above the floor, measured to the top of the seat. Seats shall not be sprung to return to a lifted position.12
Likewise, 19 inch high benches are accessible:
903.5 Height. The top of the bench seat shall be 17 inches (430 mm) minimum and 19 inches (485 mm) maximum above the floor, measured to the top of the seat.13
Nineteen-inch high bathtub seats are accessible:
610.2 Bathtub Seats. The height of bathtub seats shall be 17 inches (430 mm) minimum and 19 inches (485 mm) maximum above the bathroom floor, measured to the top of the seat. Removable in-tub seats shall be 15 inches (380 mm) minimum and 16 inches (405 mm) maximum in depth. Removable in-tub seats shall be capable of secure placement. Permanent seats shall be 15 inches (380 mm) minimum in depth and shall extend from the back wall to or beyond the outer edge of the bathtub. Permanent seats shall be positioned at the head end of the bathtub.14
Nineteen-inch high shower compartment seats are accessible:
610.3 Shower Compartment Seats. The height of shower compartment seats shall be 17 inches (430 mm) minimum and 19 inches (485 mm) maximum above the bathroom floor, measured to the top of the seat. In transfer-type and alternate roll-in-type showers, the seat shall extend along the seat wall to a point within 3 inches (75 mm) of the compartment entry. In standard roll-in-type showers, the seat shall extend from the control wall to a point within 3 inches (75 mm) of the compartment entry. Seats shall comply with Section 610.3.1 or 610.3.2.15
Nineteen-inch high amusement park rides are accessible:
1102.5.2 Transfer Height. The height of amusement ride seats designed for transfer shall be 14 inches (355 mm) minimum and 24 inches (610 mm) maximum measured from the surface of the load and unload area.16
As mentioned above, a minimum standard of 19 inches at the highest point of a table or chair is not the same as the transfer surface height experienced by patients because the front edge of currently available tables is lower than the highest measured point. However, the fact that a 19 inch height is so widely accepted by existing accessibility standards for various types of benches and chairs strongly commends maintaining the standard for medical diagnostic equipment as the minimum standard for accessible examination tables and chairs. This is particularly the case because of the manner in which medical diagnostic equipment is used. Toilets, bench seats, and bathtub seats remain at a fixed height for both transfer and normal use; in fact, usability of these devices may require constant contact of one’s feet with the floor for stability or require free use of one’s hands. Medical examination tables and chairs, on the other hand, are lowered to a low height to facilitate transfer, then are raised to a considerable height to facilitate medical examinations by the medical clinician.17
Finally, it is important to note the requirements for tolerancing under current regulations:
104.1.1 Construction and Manufacturing Tolerances. All dimensions are subject to conventional industry tolerances except where the requirement is stated as a range with specific minimum and maximum end points.18
In practical terms, because the low adjustable height is intended to be specified as a maximum dimension, manufacturers of accessible MDE will design their equipment not to exceed this maximum. Thus equipment designed to this standard will be, on average, less than the maximum allowed low adjustable height (e.g. 19 inches) by a height of conventional industry tolerances. These tolerances can be 1/4" or more, depending on the type of equipment.
NOTES
11. Although the current accessibility standards and regulations described here reference a fixed height, the MDE advisory committee has recommended adding adjustable height to further enhance accessibility for users whose WMD’s are higher than 19 inches. This includes most power wheelchair and scooter users, as described in the AWM Project. Note that the accessibility standard for pool lifts, 1009.2.4, also specifies adjustability, but with a very different range of motion due to its intended use of lowering a person down into a body of water.
12. See Accessible and Usable Buildings and Facilities, ICC/ANSI A117.1-2009. Emphasis of upper dimension of range added.
13. Ibid.
14. Ibid.
15. Ibid.
16. Ibid.
17. Because medical table and chair are typically raised in height for a clinical examination, the transfer supports described in the proposed standards may provide the added benefit of patient security and stabilization.
18. See Accessible and Usable Buildings and Facilities, ICC/ANSI A117.1-2009.
AN INCREASING NUMBER OF HEALTH CARE PROVIDERS ARE TRANSITIONING TO ADJUSTABLE HEIGHT TABLES
In 2012, approximately 25 percent of examination tables sold in the U.S. are at about a 19-inch low height.19 This is a significant increase over the 17 percent of adjustable height tables sold in 2005. While manufacturers of tables and chairs understand that lower heights may be desirable, there currently are no commercially available examination tables or chairs that can reach a height lower than 19 inches uncompressed at its highest point on seating surface. It is unclear when such a table or chair could be available.
If adopted by the Access Board, a recommendation of 19-inch maximum height will build on the growing percentage of providers voluntarily purchasing accessible, adjustable height tables, at 25 percent today.20 Conversely, if a new standard lower than 19 inches is established, thereby deeming all current adjustable tables inaccessible, the entire U.S. health system will be forced to begin at 0 percent accessible.
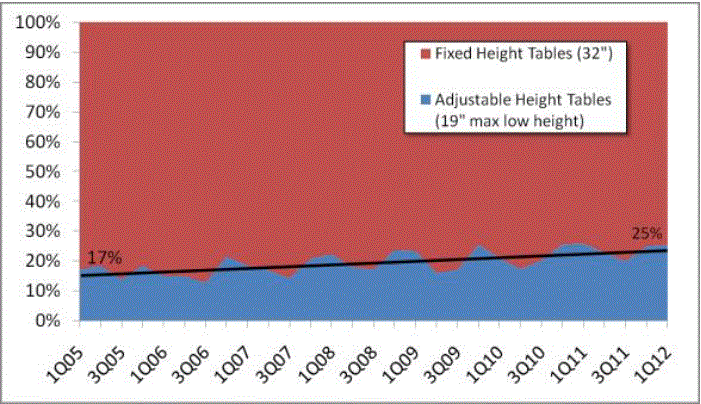
Figure 4: Sales percentages of fixed height versus adjustable height medical tables
(representing sales volume of new medical tables)
NOTES
19. Medical table install base derived from U.S. medical distribution sales data, as provided by Global Healthcare Exchange (GHX).
20. Some tables may require installation of a modified top to meet the 19” standard but would not require changing out the installed base.
19. Medical table install base derived from U.S. medical distribution sales data, as provided by Global Healthcare Exchange (GHX).
20. Some tables may require installation of a modified top to meet the 19” standard but would not require changing out the installed base.
AVAILABLE DATA DO NOT SUPPORT DEPARTING FROM THE CURRENTLY ACCEPTED STANDARD OF 19-INCH TRANSFER SURFACE HEIGHT
The Medical Diagnostic Equipment Technical Advisory Committee appropriately tried to determine what the optimal accessible transfer surface height is based on available data. In particular, the Advisory Committee spent a great deal of time discussing the Anthropometry of Wheeled Mobility (AWM) Project.21 In that study, which the U.S. Access Board commissioned, Dr. Steinfeld served as the lead investigator. This study measured the physical characteristics of people who use wheeled mobility devices and some of the characteristics of those devices. The resting position of a person before attempting an independent transfer is important, and the Committee members valued the study’s useful information in evaluating potential recommendations. However, because the Wheeled Mobility Anthropometry project only studied static positioning of users in their devices, it did not identify optimal transfer surface heights, and did not assess the ability of wheeled mobility device users to transfer independently from their mobility device onto an examination table or chair.
In evaluating data about seat height, the Committee took into account the relevant transfer height from the wheelchair. The AWM Project measured the rear compressed seat height of the wheeled mobility device with the user seated in it. For transfer purposes, however, the most relevant height is the wheelchair front edge. The height of the front edge of the seat or cushion is important because the user will shift to the front of the seat (to avoid having to lift over the side wheel) before moving to the side to complete the transfer. Unfortunately, the AWM Project did not measure the height of those same users' front wheelchair edges.
There is a recognized international standard defining various measurements of wheelchairs: ISO 7176-7:1998 Wheelchairs — Part 7: Measurement of seating and wheel dimensions. Figures 6 and 7 below are selected screenshots from this international standard. These illustrations identify several key measurements. The most important illustrations for present purposes are "seat plane angle," "effective seat depth," and "seat surface height at the front edge." The standard focuses on measurement procedure so it does not prove any actual measurements. However, as the images below make clear, the seat reference plane and effective seat depth will dictate the difference between the seat surface height at the front edge and the height of the seat at the rear of the wheelchair. Note further that wheelchair height measurements generally do not take into account the height of the cushion, which the consumer will need to clear at the front edge to enable a successful transfer.
In addition, section 4 of the Paralyzed Veterans of America’s “Guide to Wheelchair Selection”22 also illustrates the distinction between the following heights:
…the seat surface height at the front edge (which excludes the effect of a seat cushion, typically measuring 2-4" in depth), the seat height at the rear of the seated surface, and the relevant transfer surface height for clearing the seat cushion.23
Furthermore, the PVA’s “Clinical Application Guide to Standardized Wheelchair Seating Measures of The Body And Seating Support Surfaces” defines the seat surface height as “the distance from the floor to the top of the seat at front edge, in area intended for thigh loading.” It goes on to state that:
This measure is clinically relevant because it impacts the user’s overall sitting height, clearance under tables, clearance of foot supports above casters or ground, and functional activities such as transfers.25
Therefore, it cannot be inferred that the seat surface height at the front edge is the same as the seat height measured in the AWM Project. Similarly, these illustrations used in measuring individuals for manual wheelchairs indicate that the AWM Project’s data cannot be used to make a direct assessment of the table height needed to accommodate wheelchair users effectively. Based on figures 5-7 below, a 17-inch rear compressed height measured in the AWM Project could easily correspond with a 20- or 21-inch uncompressed seat surface height at the front edge. This could in turn mean that individual users participating in the AWM Project measured below 19 inches would be able to transfer comfortably to a table surface for which the highest uncompressed surface is 19 inches.
Note further in figure 5 that the rear seat height is considerably lower than the height of the wheelchair wheels, which typically measure 22-26 inches. Any transfer from the rear of the chair would require the user to transfer up and over the wheelchair wheel, again, well above a minimum low-range height of 19 inches. Based on this difficulty of transferring over the wheelchair wheel, Committee members noted transferring over the wheelchair wheel is an extremely unlikely scenario. This emphasizes the fact that the front edge of the seat is the most relevant for transfer.
In addition, a second study commissioned by the U.S. Access Board and evaluated by the Advisory Committee (the Pittsburgh study26) determined that manual wheelchair-users, who are generally the users most likely to have the lowest seated heights, are generally able to accommodate a 2-inch difference in height between one’s wheelchair and a transfer surface. Consequently, even if one posited that the AWM Project finding of 17 inches at the rear of the seat compressed corresponded to an uncompressed height of 17 inches at the front edge of the wheelchair, the maximum height of 19 inches at the highest point of a table or chair transfer surface would still fall within the 2-inch differential identified by the Pittsburgh study as accessible to most manual wheelchair users. Of note, Committee members pointed out that the Pittsburgh study utilized a sample of younger and more active subjects than the AWM Project. As such, broad conclusions for a broader population of persons with disabilities, and direct correlations with the AWM Project, should be avoided.
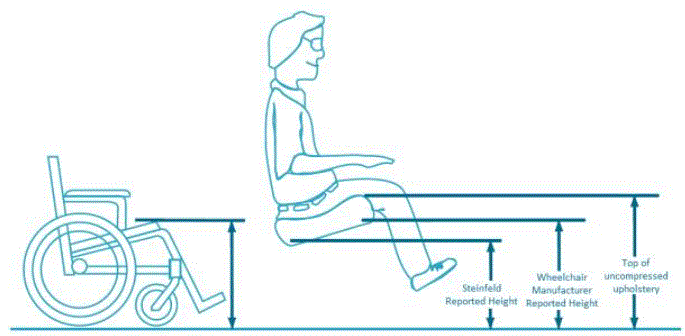
Figure 5: Adapted diagram from the “Paralyzed Veterans of America’s Guide to Wheelchair Selection” showing difference in measured height between the AWM Project, wheelchair manufacturer’s height per ISO 7176-7, and the uncompressed upholstery measurement height.
Figure 6: Wheelchair manufacturer’s height and seat construction types (showing variation in compression when person’s weight is applied) per ISO 7176-7
Figure 7: Wheelchair manufacture standardized measurements per ISO 7176-7
NOTES
21. See Analysis of Seat Heights for Wheeled Mobility Devices at: http://udeworld.com/analysis-of-seat-height-for-wheeled-mobility-devices. The seat heights ranged from 16.3 inches to 23.9 inches for manual wheelchair users; 16.2 inches to 28.9 inches for power wheelchair users; and 18.8 inches to 25.3 inches for scooter users. Seat heights for males were typically higher than for females. Thirty (30) percent of male manual wheelchair users and 6 percent of male power wheelchair users had seat heights equal to or less than 19 inches. All the male manual wheelchair users and 92 percent of the male power wheelchair users had seat heights equal to or less than 25 inches. Thus, transfer surfaces that are adjustable from 17 inches minimum to 25 inches maximum during patient transfer accommodate significantly more patients who use mobility devices.
22. Available at http://www.wheelchairnet.org/WCN_ProdServ/Docs/PDF/AXbook_Sec4a.pdf.
23. Ibid.
25. Ibid.
26. Human Engineering Research Laboratories, University of Pittsburgh, The Impact of Transfer Set-Up on the Performance of Independent Transfers: Final Report. Available at: http://herl.pitt.edu/ab/transfer_assessment_report.pdf (visited May 22, 2013).
ADOPTION OF A 19 INCH HEIGHT MINIMIZES COSTS TO HEALTH CARE PROVIDERS
In the absence of Committee consensus commending a departure from the existing broadly accepted transfer surface height maximum of 19 inches, information related to costs is especially critical to take into account in determining the minimum standard height for medical examination tables and chairs. We (representing medical table and chair manufacturers) estimated the costs of various examination table heights under consideration by the Medical Diagnostic Equipment Technical Advisory Committee using third-party data that represents approximately 80 percent of all distributed examination tables sold in the United States.27 This data was presented to the MDE Advisory Committee Meeting held on February 26 and 27, 2013.28
To determine the total number of examination rooms in the United States, we reviewed the CDC National Ambulatory Care Survey, and found that there are approximately 639,000 exam rooms in the United States today.
From this total number, and based on historic trends, we estimated that physicians will build new, or remodel existing, exam rooms at a rate of 4 percent each year, but the total number of examination rooms will remain unchanged from year to year.
Also based on historic trends, we estimated that physicians will replace equipment in 7 percent of their examination rooms each year, including new and replacement medical tables.
We also estimated that the average annual inflation rate will be 3% over the next ten years.
NOTES
27. Medical table install base derived from U.S. medical distribution sales data, as provided by Global Healthcare Exchange (GHX), found at http://www.ghx.com/product-pages/solutions/supplier-solutions/sales-data-analytics.aspx
28. The full meeting minutes, as well as a copy of the presentation delivered, can be found in Appendices A and B to this report.
BENEFITS AND COSTS: OVERVIEW
Based on our analysis, we determined that transfer surface height requirements lower than 19 inches would increase the cost of designing and manufacturing examination tables, reduce the rate of adoption of accessible equipment, and increase the health provider’s cost of purchasing accessible equipment.
While we estimate that adopting a lower-range requirement of 19 inches, health care providers will experience a 24 percent price increase. This means that a $5,000 adjustable-height examination table purchased today would cost approximately $6,200 after the Access Board adopts the requirements for knee-crutches and transfer supports because health care providers will need to retrofit existing tables and chairs with the required equipment. The price of that same table would be 39 percent higher than the cost of today’s examination tables if the height standard were lower than 19 inches.
COSTS TO LOWER MINIMUM TABLE HEIGHT
To be useful for its intended purpose, examination tables and chairs must maintain a high height of at least 32 inches, so that the health care provider has clinical access to the patient. In fact, 37 inches is the standard high height today for OB/GYN examinations. The need to reach heights above 32 inches interferes with efforts to lower the transfer surface height. The mechanical system necessary to raise the table are located in the base of the table, beneath the seating surface. As the difference between the required low-height and the necessary high-height for health care providers increases, the mechanics necessary to achieve that adjustable range become more complex and expensive.29
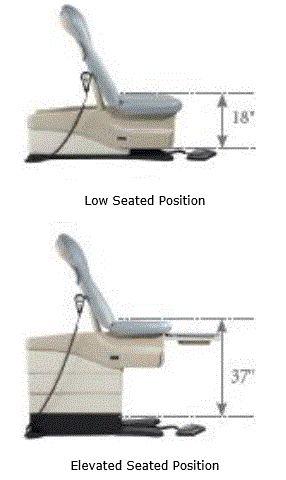
Figure 8: Range of medical table heights
In addition, we took the costs of other requirements that the Access Board is likely to adopt into account. Based on currently marketed products, we estimate the following costs:
-
Transfer Supports (TS): $500 - $1,000 (M301.3.1)
-
Leg Supports (LS): $700 - $1,100 (M301.3.2)
Therefore, the average “upgrade” cost to add transfer and leg supports would be approximately $1,650.
NOTES
29. The example given here describes an examination table, but the complexity and cost would similarly increase for examination chairs, especially those equipped with footplates. See section 4.1.3 of the full committee report for further details.
SCOPING SCENARIOS: RANGE OF POSSIBILITIES
We do not know what mandates may be put in place in the future to require Access Board standards. However, the U.S. Access Board’s decisions regarding technical requirements for equipment will significantly affect the costs on health care providers and examination table and chair manufacturers.
To illustrate this effect, we considered a broad range of scenarios. For example, if a national mandate were to require one accessible table per physician work area of five exam rooms (a typical physician practice set-up today), that would require a 20 percent adoption rate. We also considered a 100 percent adoption rate to show the full range of potential costs.
If the market adoption rate of adjustable tables were to match a requirement that one table of every five tables meet a 19 inch lower adjustable range when facility construction occurs, there would be a drop in patient access to compliant examination tables of 17% and a savings of $420 million over ten years. However, if the market adoption rate were to match a requirement that ALL new tables meet a 19-inch lower adjustable range when new construction or remodeling occurs, then accessibility would increase by 17% at a cost to health care providers of $1.38 billion over a ten-year period.
By contrast, if the market adoption rate matched a requirement to make one table of every five tables meet an adjustable range lower than 19 inches, there would be a reduction in availability of accessible tables by 35% for a savings of $530 million over ten years. However, if the requirement were to make ALL new tables meet an adjustable range lower than 19 inches when new construction or remodeling occurs, then accessibility would decrease by 1% at a cost to health care providers of $1.52 billion over a ten-year period.
The table and chart below illustrate these examples.
Table 2: Costs of Scoping Scenarios
BENEFITS AND COSTS: SUMMARY
Therefore, while establishing these requirements will result in significant costs to health care providers, establishing a lower adjustable height requirement of 19 inches will maximize the percentage of accessible tables and will cost less than requiring an adjustable height lower than 19 inches.
HOW TO MEASURE TRANSFER HEIGHTS IS IMPORTANT
Many medical examination rooms are equipped with fixed height examination tables, with a typical 32-inch seat height. However, these fixed heights tables do not allow for independent transfer of patients who use wheeled mobility devices (WMD). To solve this problem, manufacturers have designed adjustable height examination tables to work with a variety of WMD’s. Manufacturers designed the shape of the seat for these tables to meet both patient accessibility and clinical needs, resulting in a complex, contoured shape.
Because of these complex shapes, it is necessary to create a standard method by which to measure table seat dimensions. These proposed measurement techniques would apply equally to tables (M301) and chairs (M302).
FEATURES
Several necessary features determine the shape of an examination table seat:
The perineal cut-out provides access to the perineum for gynecological and urological examinations.
The corner radii allow for closer wheelchair positioning to facilitate independent transfer by minimizing gaps. The corner radii also eliminate seams in the upholstery, which improves longevity, but more importantly also improves asepsis and infection control.
Bolsters improve patient comfort and stability when seated on table. Note that the design minimizes the bolsters at the front half of the seat in order to promote ease of transfer.
Note that these features are widely used in both tables and chairs. Beds, stretchers, and other types of equipment will have unique features that determine the shapes of their patient support surfaces.
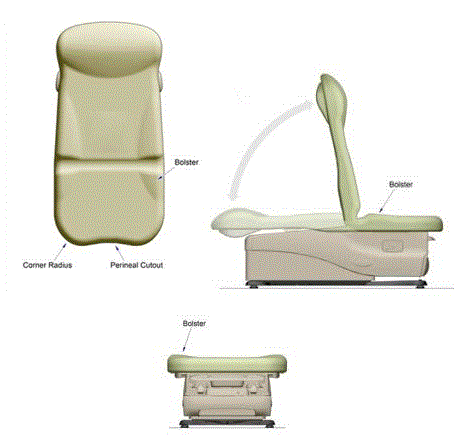
Figure 10: Features of the countered shape of a medical examination table
WMD POSITIONS FOR TRANSFER
The design of the corner radii allows closest possible position for wheelchair transfers, minimizing potential gaps and improving the patient’s ability to transfer independently.
In the diagram below, the upper wheelchair illustrates a typical side transfer, which may optionally utilize a transfer board. The lower wheelchair illustrates a typical diagonal transfer.30
Figure 11: Corner radii of a medical examination table
NOTES
30. Examples of such transfers can be viewed here: http://www.youtube.com/watch?v=qivOb_V6IgA
MEASUREMENT TECHNIQUE
The depth and width are measured along the centerlines of the seat, and the height from the floor to the highest point of the transfer surface:
-
The depth is measured between the perineal cutout and the hinge point at the back of the seat.
-
The width measured across the seat at the midpoint between the seat hinge and the front of the seat.
-
The height is measured at the highest point of the seat, inclusive of bolsters, with the foam in an uncompressed state. Note that this measurement would be at the highest point on the seating surface, which may not necessarily be at the centerline of the seat.
SEAT HEIGHT MEASUREMENT DETAIL
Regardless of measurement technique used, the height is measured at the highest point of the seat, inclusive of bolsters, with the foam in an uncompressed state. Measuring to the top of the bolster would account for the maximum transfer height differential from a wheeled mobility device.
Note that examination tables and chairs only use bolsters on certain areas of the transfer surface. Lower parts of the transfer surface (such as the front edge) would have an even lower height. For reference, ¾ inches is a typical bolster height used on many examination tables. Due to their narrower seating surfaces and the increased need to support the patient securely, examination chairs often have deeper bolsters. Dental chairs, for example, have a typical 1 ¼-inch bolster height.
CONCLUSION
We recommend that the U.S. Access Board adopt the subcommittee's original recommendations to require an adjustable range of 19 to 25 inches for the transfer surface of examination tables and chairs during patient transfers. This standard will have the most benefits for consumers with the least costs for health care providers. In addition, it will build on the growing percentage of providers voluntarily purchasing accessible, adjustable height tables.
APPENDIX A
Excerpt from the MDE Advisory Committee Meeting Minutes, February 26 and 27, 2013:31
Manufacturer Presentations
Several manufacturers presented information on technical and cost considerations for exam tables and chairs as requested at the preceding advisory committee meeting. Darren Walters, MTI and Bob Menke, Midmark Corporation led these presentations. The first presentation addressed a number of performance and efficacy considerations for examination chairs focusing on lifting mechanisms to adjust seat height, impact of height on the health care practitioner, and the relationship of exam chair footrests to seat height. The presentation revisited some of the recommendations made at the January meeting by the practitioners and clinicians about acceptable transfer surface heights. A key point was made that, while it may be technically possible for an exam chair to go even lower than what the committee is considering, there may be undesirable and unintended consequences. Benefits and limitations of the telescoping, scissor and cantilevered lift systems were reviewed. Concerns were voiced that lowering the equipment could affect the equipment effectiveness and positioning for proper exams. The committee discussed at length the different lifting systems used for adjustable height equipment and the viability of achieving the heights being considered. The presentation referred to relevant requirements for height in the ADA and ABA Accessibility Guidelines and guidance adopted by the US Department of Justice.
The afternoon presentation focused on the cost and benefit analysis based on the implementation of key provisions in the proposed Standard affecting exam tables. The presentation covered the present availability of accessible height adjustable exam tables and how this would change in the future with different scenarios based on the low heights being considered for the transfer surface. The presentation also emphasized that 18% of existing rooms in the United States are equipped with adjustable height tables that can achieve a 19 inches low height providing a head start to maximize the availability of accessible rooms at a lower cost if the 19 inches is adopted as the standard. Committee discussions were interspersed between segments of the presentation with consideration given to research methodology, the baselines used, and health care practice issues affecting the selection and purchase of diagnostic equipment. A key issue addressed in the presentation is that no adjustable height exam tables are currently available that lower to 17 inches above the floor.
Discussions during and after the presentations did not produce consensus about the minimum transfer surface height. The committee decided to refer the issue to the Exam Tables and Chairs subcommittee for further review and discussion. The full committee will then consider the Subcommittee’s recommendation.
NOTES
31. Full meeting minutes are available at http://www.access-board.gov/guidelines-and-standards/health-care/about-this-rulemaking/background/committee-meetings/minutes-february-26-and-27,-2013
APPENDIX B
Presentation given by Bob Menke, Midmark Corporation, at the February 27, 2013 Advisory Committee Meeting:






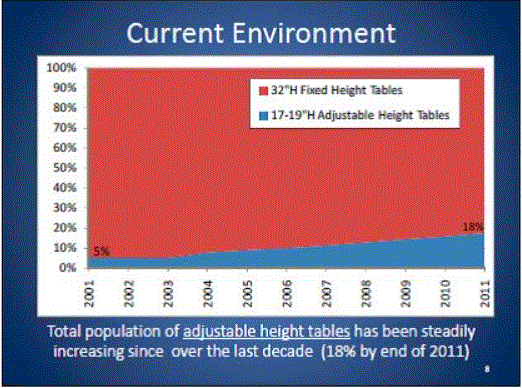
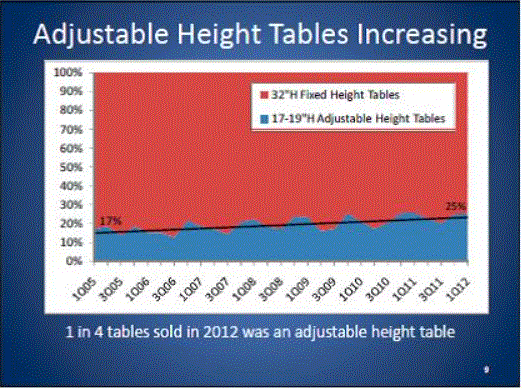
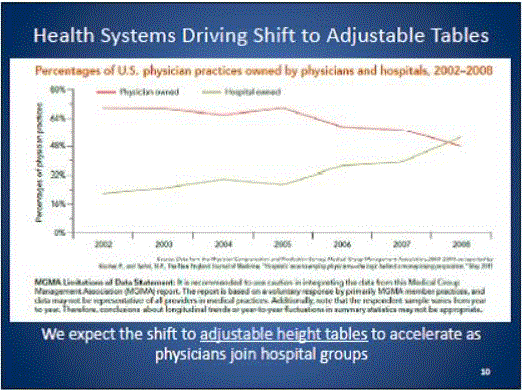


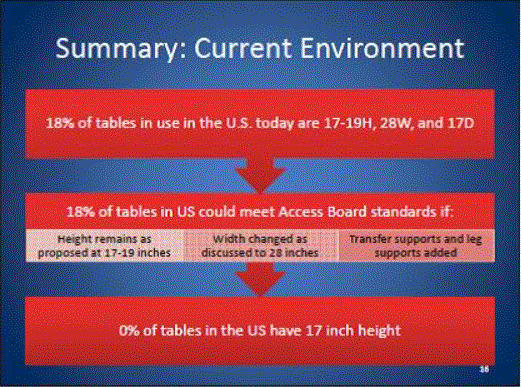
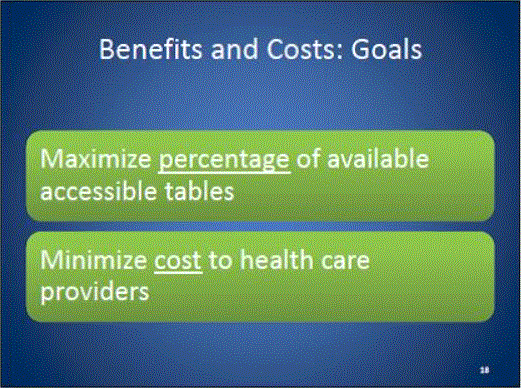

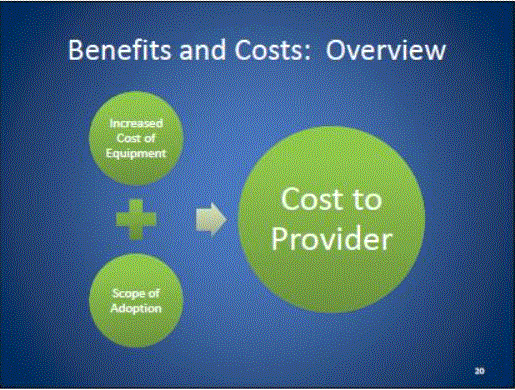
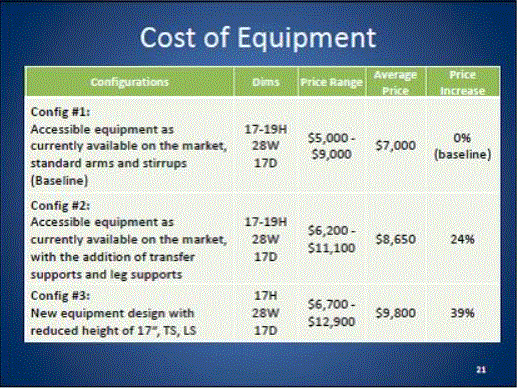


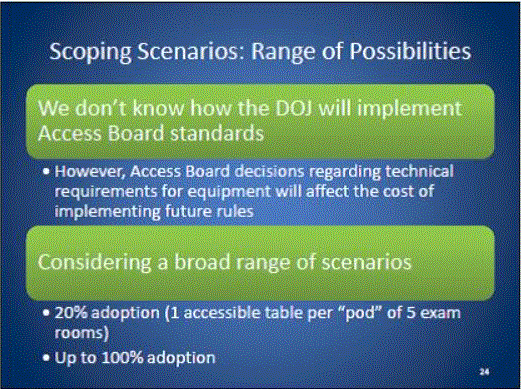
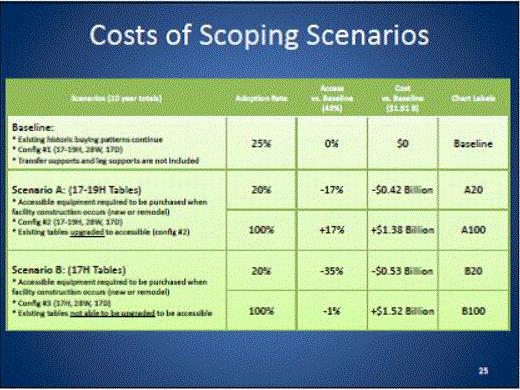
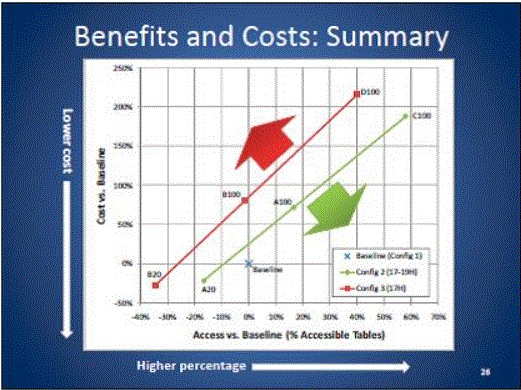
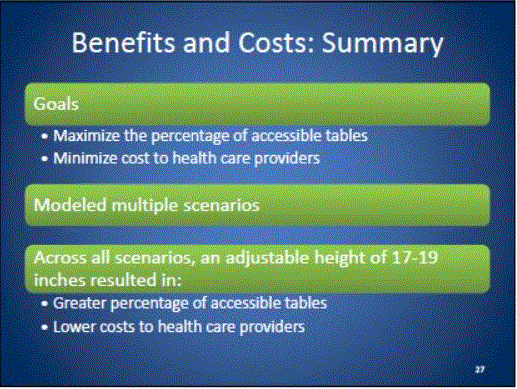
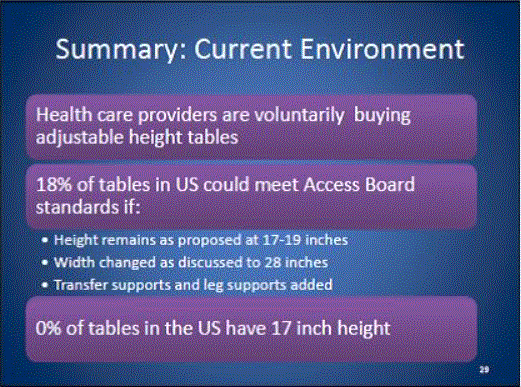
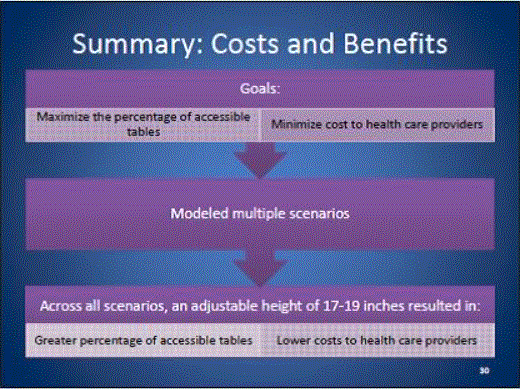
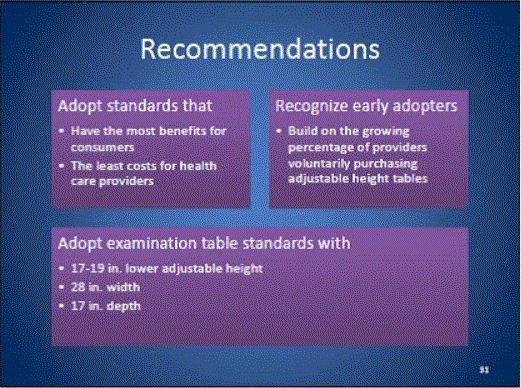

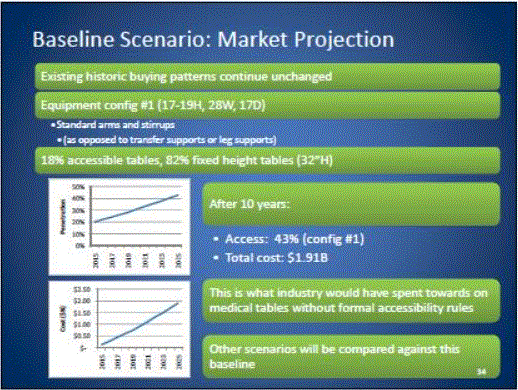
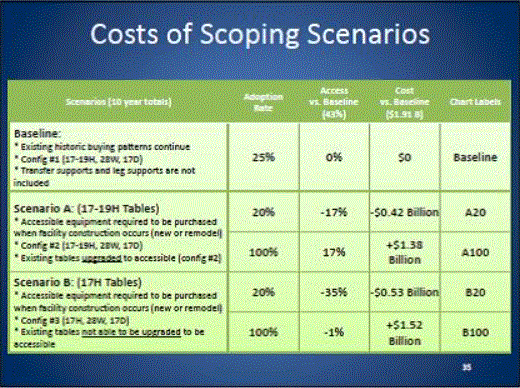
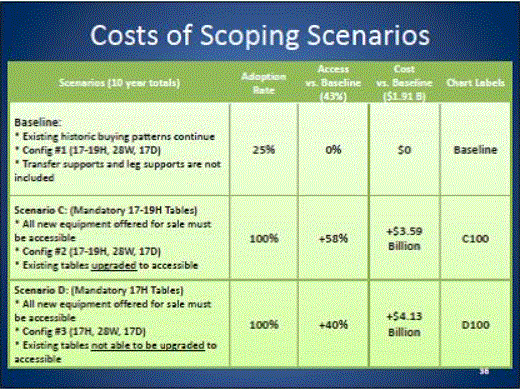
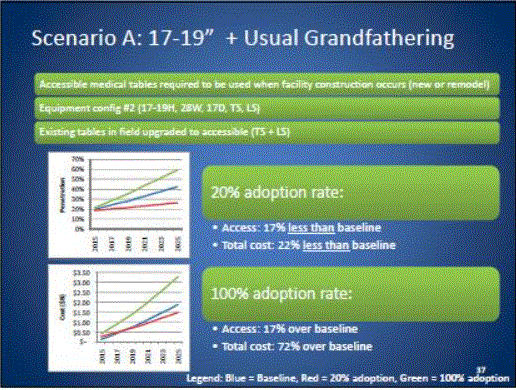
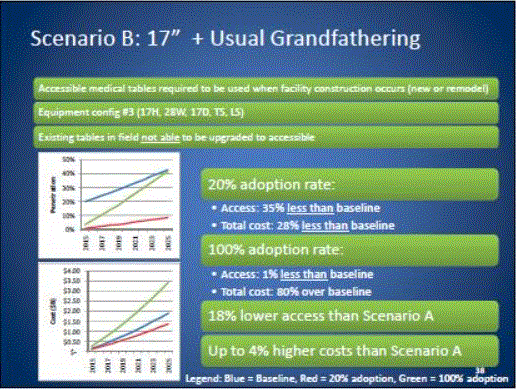
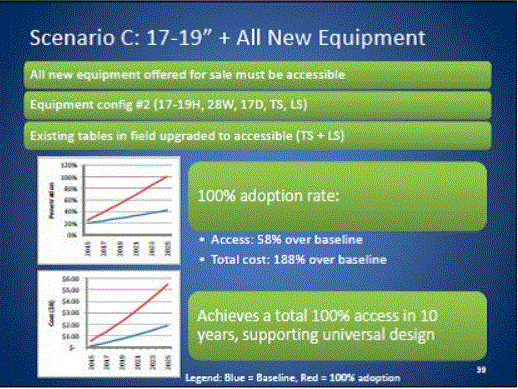
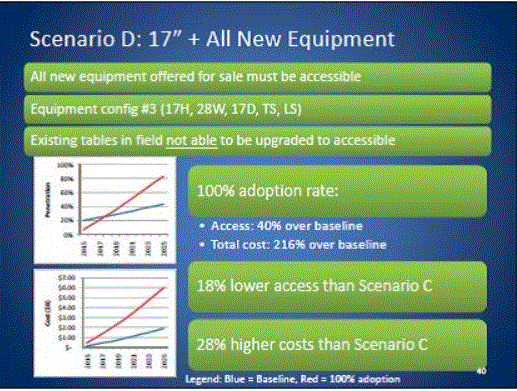
NOTES
1. Patient Protection and Affordable Care Act, Pub. L. No. 111-148, §4203, 124 Stat. 119 (2010).
2. See Architectural and Transportation Barriers Compliance Board. Notice of Proposed Rulemaking: Medical Diagnostic Equipment Accessibility Standards, 77 Fed. Reg. 6916, February 9, 2012
3. Note that height measurement, as defined by the tables and chairs subcommittee, represents the highest point of the transfer surface, inclusive of bolsters, when measuring to the top of uncompressed foam. See the “Measurements of Tables and Chairs” subcommittee report, dated April 5th, 2013.
4. Medical table install base derived from U.S. medical distribution sales data, as provided by Global Healthcare Exchange (GHX), found at http://www.ghx.com/product-pages/solutions/supplier-solutions/sales-data-analytics.aspx
5. See M301.2.1 and M302.2.1.
6. See Architectural and Transportation Barriers Compliance Board. Notice of Proposed Rulemaking: Proposed Accessibility Standards for Medical Diagnostic Equipment. February 8, 2012.
7. Ibid.
8. Ibid.
9. Ibid, citing ANSI/AAMI HE 75, section 16.4.4. ANSI/AAMI HE75 recommends that the height of patient support surfaces "should be easy to adjust (ideally, powered) to suit the needs of health care professionals and patients." ANSI/AAMI HE75 further recommends that the height of patient support surfaces "should be adjustable to a position high enough to accommodate tall health care providers and the range of medical procedures that could occur . . .[and] to a position low enough [19 inches maximum] to allow for the comfort of providers who choose to work in a seated position, to enable patients to keep their feet on the floor while seated, and to accommodate patients who need to transfer laterally between the platform and a chair or wheelchair alongside."
10. See Analysis of Seat Heights for Wheeled Mobility Devices at: http://udeworld.com/analysis-of-seat-height-for-wheeled-mobility-devices. The seat heights ranged from 16.3 inches to 23.9 inches for manual wheelchair users; 16.2 inches to 28.9 inches for power wheelchair users; and 18.8 inches to 25.3 inches for scooter users. Seat heights for males were typically higher than for females. Thirty (30) percent of female manual wheelchair users and 6 percent of female power wheelchair users had seat heights equal to or less than 19 inches. All the male manual wheelchair users and 92 percent of the male power wheelchair users had seat heights equal to or less than 25 inches. Thus, transfer surfaces that are adjustable from 17 inches minimum to 25 inches maximum during patient transfer accommodate significantly more patients who use mobility devices.
11. Although the current accessibility standards and regulations described here reference a fixed height, the MDE advisory committee has recommended adding adjustable height to further enhance accessibility for users whose WMD’s are higher than 19 inches. This includes most power wheelchair and scooter users, as described in the AWM Project. Note that the accessibility standard for pool lifts, 1009.2.4, also specifies adjustability, but with a very different range of motion due to its intended use of lowering a person down into a body of water.
12. See Accessible and Usable Buildings and Facilities, ICC/ANSI A117.1-2009. Emphasis of upper dimension of range added.
13. Ibid.
14. Ibid.
15. Ibid.
16. Ibid.
17. Because medical table and chair are typically raised in height for a clinical examination, the transfer supports described in the proposed standards may provide the added benefit of patient security and stabilization.
18. See Accessible and Usable Buildings and Facilities, ICC/ANSI A117.1-2009.
19. Medical table install base derived from U.S. medical distribution sales data, as provided by Global Healthcare Exchange (GHX).
20. Some tables may require installation of a modified top to meet the 19” standard but would not require changing out the installed base.
21. See Analysis of Seat Heights for Wheeled Mobility Devices at: http://udeworld.com/analysis-of-seat-height-for-wheeled-mobility-devices. The seat heights ranged from 16.3 inches to 23.9 inches for manual wheelchair users; 16.2 inches to 28.9 inches for power wheelchair users; and 18.8 inches to 25.3 inches for scooter users. Seat heights for males were typically higher than for females. Thirty (30) percent of male manual wheelchair users and 6 percent of male power wheelchair users had seat heights equal to or less than 19 inches. All the male manual wheelchair users and 92 percent of the male power wheelchair users had seat heights equal to or less than 25 inches. Thus, transfer surfaces that are adjustable from 17 inches minimum to 25 inches maximum during patient transfer accommodate significantly more patients who use mobility devices.
22. Available at http://www.wheelchairnet.org/WCN_ProdServ/Docs/PDF/AXbook_Sec4a.pdf.
23. Ibid.
24. Available at http://www.ucdenver.edu/academics/colleges/medicalschool/programs/atp/Resources/WheelchairSeating/Pages/WheelchairSeating.aspx
25. Ibid.
26. Human Engineering Research Laboratories, University of Pittsburgh, The Impact of Transfer Set-Up on the Performance of Independent Transfers: Final Report. Available at: http://herl.pitt.edu/ab/transfer_assessment_report.pdf (visited May 22, 2013).
27. Medical table install base derived from U.S. medical distribution sales data, as provided by Global Healthcare Exchange (GHX), found at http://www.ghx.com/product-pages/solutions/supplier-solutions/sales-data-analytics.aspx
28. The full meeting minutes, as well as a copy of the presentation delivered, can be found in Appendices A and B to this report.
29. The example given here describes an examination table, but the complexity and cost would similarly increase for examination chairs, especially those equipped with footplates. See section 4.1.3 of the full committee report for further details.
30. Examples of such transfers can be viewed here: http://www.youtube.com/watch?v=qivOb_V6IgA
31. Full meeting minutes are available at http://www.access-board.gov/guidelines-and-standards/health-care/about-this-rulemaking/background/committee-meetings/minutes-february-26-and-27,-2013



User Comments/Questions
Add Comment/Question