Recommendations on Standards for the Design of Medical Diagnostic Equipment for Adults with Disabilities, Advisory Committee Final Report
7. Diagnostic Imaging Equipment: Accessibility Considerations
Section 4 introduces the complex considerations raised when considering accessibility standards, such as transfer surface heights and transfer supports, for current diagnostic imaging equipment. As described there and detailed further below, when contemplating improving accessibility, today’s imaging technologies present certain technical constraints, some of which relate to basic laws of physics and the physical properties of equipment elements (e.g., magnets). Nonetheless, all patients regardless of disability must have access to these technologies, which can be essential to identifying and characterizing disease and thus directing critical therapeutic decisions. This imperative is not only the law, it is essential to ensuring equitable quality of care.
Early during Advisory Committee deliberations the suggestion arose of facilitating access for persons with disabilities to diagnostic imaging machines through modifying the immediate environment rather than the machine itself – through what Committee members initially called an “accessibility kit” and later an “accessibility package.” For reasons described below, in drafting this report the Editorial Subcommittee changed this language to “imaging system accessibility configuration.” This new phrase – imaging system accessibility configuration – now substitutes for accessibility package, but the two phrases represent the same concept.
The purpose of this section is to describe the rationale for imaging system accessibility configurations in the setting of today’s diagnostic imaging equipment design. The section suggests possible components of these accessibility configurations; it also raises specific cautions and concerns about this approach. Throughout it is critical to emphasize that this proposal for imaging system accessibility configurations relates to current equipment designs. Although immutable laws of physics might constrain new designs of imaging equipment that contains certain components such as magnets or x-rays, for example, future technologies might use entirely different modalities to image soft tissues, boney structures, and even microanatomy. Future technologies might provide not only breakthroughs in the science of imaging but also eliminate or minimize impediments to providing accessibility to persons with disabilities.
7.1 Basic Understanding of Diagnostic Imaging Equipment
One challenge to Advisory Committee deliberations about accessibility approaches for these technologies was that Committee members had fundamentally different understandings about what constitutes diagnostic imaging equipment. Members outside the imaging industry generally thought of the machine itself, upon which patients lie or sit and which “takes the picture,” as “diagnostic imaging equipment.” Manufacturers, however, hold a different understanding, founded upon existing FDA definitions (see below). As described in Section 4, in contrast to compact and mobile imaging technologies such as ultrasound and echocardiography machines, the imaging equipment covered by the accessibility standards is part of a system of interacting components, permanently mounted in fixed installations. Manufacturers see imaging equipment systems as an inextricably linked amalgam of the machine with its multiple technological components and accessories that generate the images and the spaces immediately surrounding the machine. These specially designed spaces must perform specific essential functions, such as supporting the equipment’s heavy weight, shielding ionizing radiation or magnetic fields, and providing specialized high power capacity electrical service. The machine that performs the imaging test does not operate in isolation; its immediate surroundings are integral to the performance and safety of diagnostic imaging equipment.
Considering diagnostic imaging equipment in this way, not as the imaging machine alone but as an integrated system with its accessories and installation, is consistent with regulations governing this equipment. As noted in Section 4.3.1, the U.S. Food and Drug Administration (FDA) classifies most diagnostic imaging technologies as Class II medical devices, most of which need FDA clearance (via a “510(k)” notification) prior to being placed on the market. In particular, Section 201(h) of the Federal Food Drug and Cosmetic Act includes accessoriesKK with the definition of a medical device.LL
During Advisory Committee and Imaging Equipment Subcommittee deliberations, considering imaging equipment from this integrated perspective led to recommendations about the development of imaging system “accessibility packages,” renamed “imaging system accessibility configurations” in this report to underscore the understanding of imaging equipment as a fully-integrated system of component parts, including its immediately surrounding space and accessories. Examples of what accessibility configurations might include appear in Section 7.3. First, however, Section 7.2 provides more details about the technological and scientific aspects of diagnostic imaging equipment, introduced in Section 4.3 that might influence efforts to provide access to the imaging machine itself.
Notes
KK The term “device” (except when used in paragraph (n) of this section and in sections 331(i), 343(f), 352(c), and 362(c) of this title) means an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including any component, part, or accessory, which is— (1) recognized in the official National Formulary, or the United States Pharmacopeia, or any supplement to them, (2) intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or (3) intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of its primary intended purposes.
LL The IEC 60601-1 international standard for medical electrical equipment indicates that medical electrical equipment includes those accessories that are necessary to enable the normal use of the equipment which includes facilitation of its use.
7.2 Technological and Scientific Considerations
As described in Section 4.3.2, some types of current diagnostic imaging equipment present serious technical, scientific, and design impediments to improve accessibility of the height of machines’ transfer surfaces, mounting of transfer supports, and/or spaces for portable lifts. For example, today’s DXA and certain x-ray systems cannot be height adjustable due to the functional operation of the equipment (Section 4.3). Two types of imaging equipment with bores offer examples of technical concerns that can affect transfer surface height and other access considerations:
-
For magnetic resonance imaging (MRI), the resolution of the images is proportional to the magnetic field strength. The magnets used for MRI scanners are usually solenoidal: a long cylinder with an inside diameter that must accommodate the patient. Most MRI devices today have magnetic fields of 1.5 T (Tesla) and greater. These large magnetic fields can only be achieved with superconducting magnets, which require cryogenics to maintain liquid helium temperatures. Such cryogenics necessarily take up space, but the precise amount of space needed is a matter of engineering. With the trend to go to larger magnetic fields of 3 T and higher to produce higher-resolution images, it is likely that the space requirements might increase in the future. Nonetheless, the Committee noted it is technically feasible for MRIs to lower to accessible heights.
-
In contrast, the imaging device of computed tomography (CT) is approximately an annulus (or ring) with an internal diameter sufficient to accommodate the patient. The imaging works by rotating an x-ray tube and detector around the annulus or 360 degrees around the patient, imaging cross-sectional slices that are arranged longitudinally. The need for the rotating imaging places engineering constraints on the size and height of the device. It is currently possible for some CT devices to lower to 18” to facilitate transfers.
Additionally the transfer surface of these imaging devices, the patient table, plays an integral role in achieving the systems’ diagnostic purpose. The tables are necessarily included in the image plane and in many cases must provide sub-millimeter positioning accuracy while being designed to still safely support very large patients (e.g. greater than 450 lbs).
Some features of imaging equipment design affecting height may change in the future. Advisory Committee members repeatedly urged the accessibility recommendations to look forward and motivate this change. To the extent, however, that future imaging technologies continue to rely on specific modalities or energy sources – such as magnetic fields and x-ray tubes and detectors – fundamental laws of nature and physics might constrain or circumscribe what is feasible in adjustability of transfer surface heights.
Other features of current imaging technology design might affect accessibility. For instance, some x-ray tables require bi-directional horizontal movement to generate the specific image required for diagnostic purposes. Many tables, especially in equipment with bores, move into and out of the bore while producing images of the anatomical region undergoing diagnostic evaluation; these tables may have both moveable and stationary components. In these situations involving current technology, supports to aid transfers might not be feasible to attach to the table for several reasons including: lack of structural integrity on the side where the support is affixed; other structural problems, such as compromised tubing and other equipment components; risks of patients being caught in a tableside support; and impeded access of radiology technicians and other clinical staff to patients during the imaging study, such as for administration of imaging agents, providing other drugs, and monitoring patients.
7.3 Imaging System Accessibility Configuration Examples and Concerns
The overall goal of imaging system accessibility configurations is to offer technically feasible, relatively low cost solutions to improving accessibility of existing imaging equipment and thus meet recommended standards for diagnostic equipment accessibility. These accessibility configurations could possibly be considered as integral components of diagnostic imaging equipment, just as are other accessories under FDA regulations. As noted above, the concept of imaging system accessibility configurations arose initially from deliberations of the Diagnostic Imaging Subcommittee, and the Advisory Committee discussed this possibility at length. Making specific recommendations about the components of this new concept was beyond the scope of the Advisory Committee. Nonetheless, given the interest of some Advisory Committee members in this option, this section of the report provides background information and preliminary concepts about potential imaging system accessibility configurations.
Members of the Diagnostic Imaging Subcommittee suggested a number of possible imaging system accessibility configurations. Examples of potential configuration components include:
-
Making architectural changes to sites where equipment is installed, such as embedding parts of the equipment into the floor (or raising the room floor). This would reduce the distance between the floor and the transfer surface.
-
Installing ramps or scissor-lifts next to equipment to raise wheelchair users closer to the transfer surfaces.
-
Installing adjustable/collapsible handgrips next to the transfer surface to provide patient transfer or positioning support.
-
Using ceiling-mounted lifts to raise the patient onto the table if there is not sufficient space to accommodate a portable lift (e.g., because of inadequate room underneath the equipment’s base for a portable lift to be positioned).MM
Three figures provide images of potential imaging system accessibility configurations:
Figure 7.3(a). Illustration of a concept (not to scale) for a detachable floor mounted support. The support could be made to be both height adjustable and detachable at floor level.
(SOURCE: GE Healthcare)
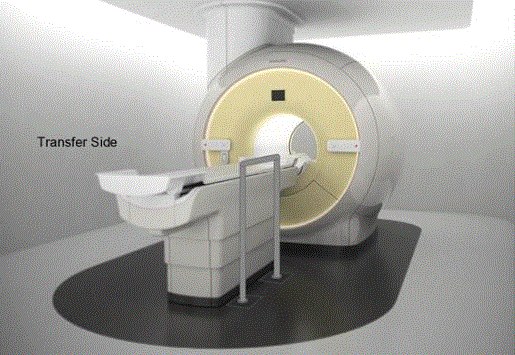
Figure 7.3(b). Illustration of a concept (not to scale) for a wheeled support. The wheels would lock and the base is sufficiently robust and sized for appropriate loadings. The support could be made to be height adjustable.
(SOURCE: GE Healthcare)
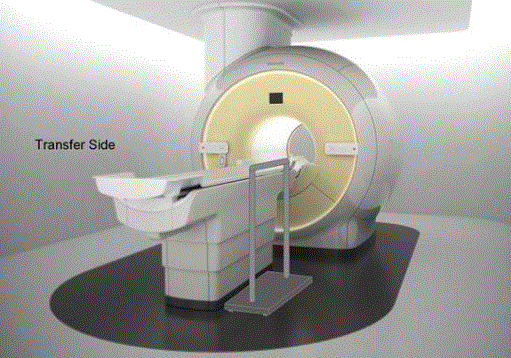
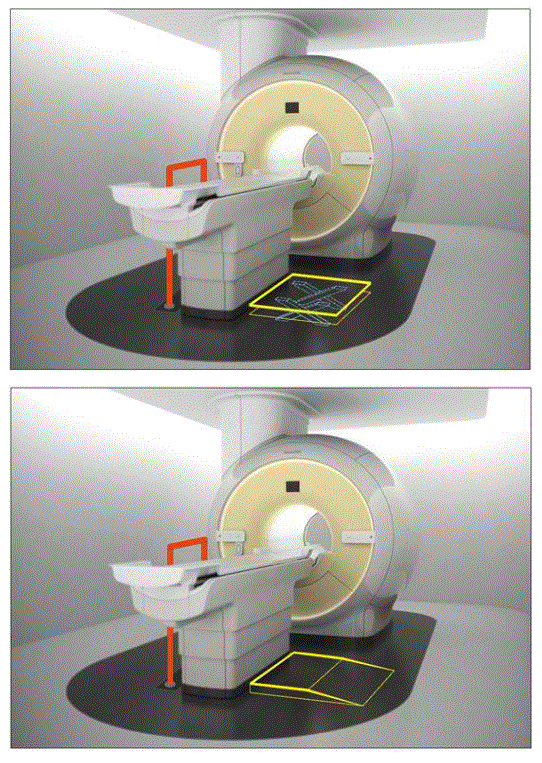
Figure 7.3(c). Illustration of the concept (not to scale) of various accessories deployed as part of an accessibility configuration. The first illustration shows a floor mounted support combined with a scissor lift. The following illustration shows the floor mounted support combined with the elevated platform.
(SOURCE: GE Healthcare)
Some committee members expressed concerns about various aspects of the imaging system accessibility configuration concept and examples. One issue involved the practical implications of some components of these suggested accessibility configurations. For example, a detachable handhold might not be readily available to patients (i.e., because they are detachable, the handhold might have been moved elsewhere). Technicians might not be trained adequately in the use of these accessibility aids. Lifts cannot be used independently. Embedding equipment into the floor of rooms in the imaging suite might be expensive and architecturally challenging. Therefore, some of the more “fixed” accessibility configurations might not appeal to some health care providers.
Committee members also raised concerns about potential safety issues. Having ramps built next to equipment – or scissor lifts – raised questions about safety for individuals using mobility aids and perhaps clinical personnel (e.g., because they could create tripping or falling hazards). These types of changes would require further study of risks to safety for patients and staff. In addition, these configurations would need to allow access for emergency personnel.
Again, the Advisory Committee urged the building of accessibility features into future equipment designs. But some Committee members, including certain representatives of equipment manufacturers and health care delivery systems (equipment purchasers), emphasized the reality that imaging equipment today represents a large capital outlay for health care facilities. This equipment generally is used for many years before being replaced with newer technologies. Some members of the Advisory Committee saw imaging system accessibility configurations as an option for “retrofitting” many existing systems to improve their accessibility within a relatively short time frame. Furthermore, accessibility accessories, ancillary equipment, and/or site features could be designed to have minimal impact on current imaging systems, thereby increasing the likelihood of adoption and thus the total number of accessible systems. Additionally given the costs and lengthy time involved for new equipment design, Committee representatives of imaging manufacturers indicated their belief that the imaging system accessibility configurations would provide a lower customer cost, timely strategy for improving accessibility to this equipment in the near term.
Notes
MM These two lifting methods would be viewed as equivalent.
7.4 Legal and Regulatory Considerations
The Americans with Disabilities Act and Section 504 of the Rehabilitation Act of 1973 require that covered medical care providers ensure that medical care and services are provided to persons with disabilities in a nondiscriminatory manner. These federal laws also require that persons with disabilities be given an equal opportunity to participate in and benefit from the providers’ medical services, including access to the facilities where services are provided and to the equipment medical practitioners’ use for diagnostic and other services. Accessible medical equipment is required in order to meet this nondiscrimination obligation and eliminate the barriers that inaccessible equipment can create for persons with disabilities. In cases where imaging equipment is fixed, it must meet the requirements of the 2010 ADA Standards. The requirements imposed by these Standards, which are enforceable under the ADA, were only peripherally considered during Advisory Committee’s deliberations. Regulations implementing these federal laws do not currently include specific technical requirements for the accessibility of non-fixed medical equipment, although steps are underway by the Department of Justice (DOJ) to propose specific ADA technical standards for medical equipment.
DOJ’s future work on medical equipment will be broader in scope than the Access Board’s work as it will not be limited to diagnostic medical equipment. In addition, later DOJ regulations may lay out limited circumstances where “equivalent facilitation” is permissible under the Americans with Disabilities Act. For example, later regulations may discuss possible limited circumstances in which alternative means may be used to provide equivalent or greater access to medical equipment through the use of ancillary equipment, lifts, or architectural modifications in an interim period before medical equipment with integrated accessible features is fully available
FDA may review imaging equipment that has been modified to make it accessible when a manufacturer wants to introduce it into commercial distribution in the U.S. for the first time, or if a marketed device has been changed in a way that could significantly affect the device’s safety or effectiveness. FDA reviews most imaging equipment as Class II medical devices because they typically are substantially equivalent to other equipment that is already approved and on the market. If manufacturers make substantial changes to or new claims about Class II devices, then they might need to submit materials to FDA to show that the modified product meets the standard of “substantial equivalence”.NN If the manufacturer states in its labeling that the device is “accessible” because it conforms to the new standards, this would be a marketing claim that FDA would review to verify that the claim is accurate, for example, by verifying the device’s range of height adjustability. Only very few technologically innovative products that present high levels of risk and have no substantially equivalent predicate on the market are required to go through FDA’s more stringent Class III device premarket approval (PMA) process to assure their safety and effectiveness.OO In addition, FDA exempts several categories of common medical devices, including most radiological tables, examination tables, and scales, from any FDA clearance or approval requirement.
Notes
NN It is important to note that the ADA concept of “equivalent facilitation” and the FDA concept of “substantial equivalence” are not identical or interchangeable.
OO It is extremely unlikely that modifying any imaging equipment to make it accessible would trigger a premarket approval process.
Notes
KK The term “device” (except when used in paragraph (n) of this section and in sections 331(i), 343(f), 352(c), and 362(c) of this title) means an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including any component, part, or accessory, which is— (1) recognized in the official National Formulary, or the United States Pharmacopeia, or any supplement to them, (2) intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or (3) intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of its primary intended purposes.
LL The IEC 60601-1 international standard for medical electrical equipment indicates that medical electrical equipment includes those accessories that are necessary to enable the normal use of the equipment which includes facilitation of its use.
MM These two lifting methods would be viewed as equivalent.
NN It is important to note that the ADA concept of “equivalent facilitation” and the FDA concept of “substantial equivalence” are not identical or interchangeable.
OO It is extremely unlikely that modifying any imaging equipment to make it accessible would trigger a premarket approval process.

User Comments/Questions
Add Comment/Question