Recommendations on Standards for the Design of Medical Diagnostic Equipment for Adults with Disabilities, Advisory Committee Final Report
2.4 MDE Manufacturers and Accessibility
2.4.1 Current Marketplace Dynamics
The Americans with Disabilities Act and Section 504 of the Rehabilitation Act of 1973 already require that covered medical care providers ensure the provision of medical care and services to persons with disabilities in a nondiscriminatory manner. These federal laws also require that persons with disabilities have an equal opportunity to participate in and benefit from the providers’ medical services, including having access to health care facilities and to the medical equipment used to provide services. Accessible medical equipment is required in order to meet this nondiscrimination obligation and eliminate the barriers that inaccessible equipment can create for persons with disabilities. As is discussed below, the regulations implementing these federal laws do not currently include specific technical requirements for the accessibility of non-fixed medical equipment, although steps are underway by the Department of Justice to propose specific ADA technical standards for medical equipment.
Even without specific technical requirements for non-fixed medical equipment, the examination table marketplace is developing and selling more equipment intended to meet accessibility needs. As noted above, historically, fixed-height examination tables set at 32” for the convenience of clinicians have dominated the health care delivery system. Increasingly, these so-called “box tables” are being replaced by examination tables that are height adjustable, such as that shown in Figure 2.4.1(a). Typically, height adjustable tables today lower to about 19” from the floor.I Sales figures from examination table manufacturers indicate that in 2012, about 25% of examination tables sold are height-adjustable (Figure 2.4.1(b)).
Other MDE manufacturers have also begun addressing accessibility issues, primarily within certain specialized populations. For example, manufacturers of diagnostic imaging equipment have produced equipment specifically to serve pediatric populations and persons with extreme obesity (so-called “bariatric” equipment).J Diagnostic imaging equipment manufacturers are also designing equipment for specific health care delivery settings, including facilities with lower financial resources (e.g., institutions in rural regions, developing countries) and hospital emergency departments. As part of designing for broader patient populations and delivery settings, new equipment designs incorporate patient and clinician usability considerations, such as adjustable table heights and table minimum heights.
Figure 2.4.1(a)
Height Adjustable and Fixed-Height “Box” Examination Tables
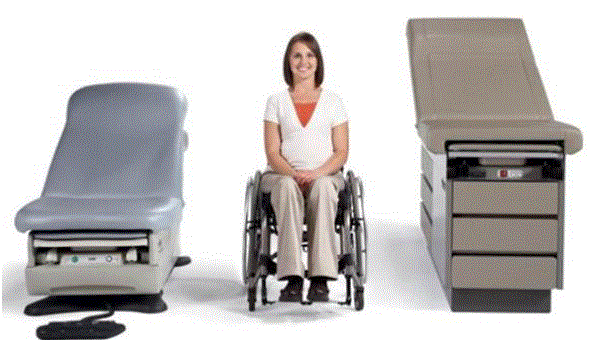
(SOURCE: Midmark Corporation) Fixed height table on right, adjustable height table on left.
Figure 2.4.1(b)
Types of Examination Tables Sold: 2005-2012
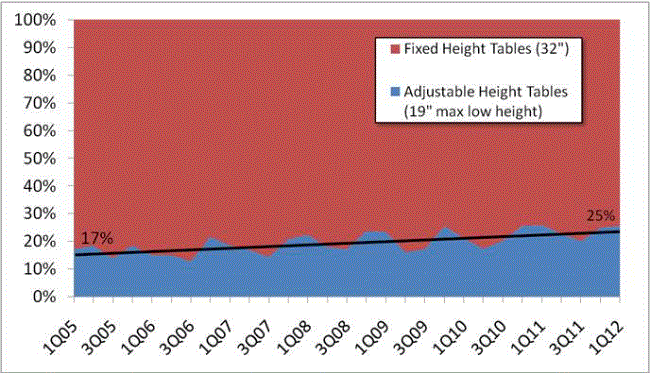
(SOURCE: Global Healthcare Exchange (GHX)) The y axis indicates the percent of tables sold that are adjustable height (blue) and fixed height (red). The x axis indicates the quarter and year of the data (e.g., first quarter in 2005, extreme left).
Notes
I The methods for measuring “low heights” for current height adjustable products vary among manufacturers and product designs. No official measurement method currently exists. Sections 4.1.2 and 5.1.4 describe the new standardized measurement method proposed by the Advisory Committee. Heights referenced in this report utilize this proposed new measurement method.
J As noted in Sections 1 and 8, this report does not address accessibility standards for children or for individuals with extreme obesity.
2.4.2 Considerations of Manufacturers in Accessible MDE Design
MDE plays a central role in ensuring the health and well-being of all individuals by supporting the detection of diseases and disorders – essential information for developing treatments or therapeutic regimens to cure, control, or significantly palliate a wide range of health problems across the life span. Therefore, an immutable core attribute of MDE must be its ability to effectively support accurate and timely diagnoses. Other key factors that guide MDE manufacturers in their equipment design include: the nature of the specific diagnostic objective; safety requirements; FDA regulations (specifically 21CFR Part 820 – Quality Systems Regulations) and processes (Section 2.5); expected patient and user demographics; international standards for safety, essential performance, and usability; ergonomic guidelines; and ultimately, validation with representative customers to help establish the safety and effectiveness of the medical devices. MDE manufacturers believe that their usability requirements should include some measure of accessibility.
As detailed in Section 2.5.2.1, the most common standard used by manufacturers to guide the design of medical equipment is ANSI/AAMI ES60601-1:2005K, which is the U.S. version of the larger scope of the International Electrotechnical Commission’s IEC 60601 series of standards for basic safety and essential performance of medical electrical equipment which also must be met. Imaging equipment that utilizes ionizing radiation must also comply with 21CFR Subchaper J. As detailed further in Section 2.5, all medical devices must adhere to FDA quality system regulation (21CFR820), applicable pre-market notification or approval processes, and risk management performed in accordance with ISO 14971.
These standards, regulations, and recommended practices provide details concerning the elements supporting the basic safety and essential performance of MDE. Standards with particular relevance to accessibility considerations include:
-
Instability hazards: including the risk of the equipment tipping. Avoiding dangerous tipping is particularly important as patients transfer onto or off of MDE or reposition themselves upon it. As the width of MDE, such as examination tables and chairs increases, equipment may require redesign to address changes in tipping hazards. To mitigate tipping risks, the size of table and chair bases can be increased. This increase in base size can affect lift capability, reduce the open areas around equipment in the examination room, and require significant product redesigns (e.g., to ensure lift compatibility).
-
Patient support safety factors: Patient support devices must meet applicable safety factors as delineated in IEC 60601-1. These factors typically range from 4x to 8x. This means a patient table labeled to support a 500 lb. patient must actually be designed and tested at up to 4,000 lbs. This has significant implications for adjustable height table design as many designs lose mechanical advantages as they go lower.
To guide their design efforts, manufacturers rely on anthropometric information,L such as the specific measurements of men and women from the 5th to 95th percentiles. They also must consider the use of the MDE for the operator, including accessibility for the operator, topics which are not addressed in the NPRM. For example, accessibility standards could define operator requirements for communication (M306) and operable parts (M307). This is particularly important regarding standards for proper ergonomics for lifting and bending, which should meet Occupational Safety and Health Administration (OSHA) Standards (Section 2.3).M
For clinical personnel, as the size of the transfer surface increases, the likelihood rises of putting clinical personnel into an unfavorable ergonomic position as a result of increasing their need to reach, lean or stretch. OSHA standards speak directly to this issue of ergonomic hazards within health care, including specific concerns about awkward postures and patient handling.N
Another critical factor that must be considered is the ability of clinical staff to access the patient during a diagnostic imaging exam to ensure proper positioning, administer imaging agents or other drugs, monitor the patient, respond to patients’ requests, and so on. Additionally, because in most cases, the tables on diagnostic imaging equipment move during the exam; tables may have both moveable and stationary parts. Therefore, design consideration must be given to avoid tubing and other such items that are attached to the patient from getting caught in a tableside support and pulled out of a patient with the potential for injuring patients, compromising image quality, or necessitating another imaging procedure.
Notes
K Association for the Advancement of Medical Instrumentation. ANSI/AAMI ES60601-1:2005 (R) 2012. Medical Electrical Equipment—Part 1: General Requirements for Basic Safety and Essential Performance. Approved 9 February 2006 by American National Standards Institute, Inc. Revised 2012.
L Tilley, Alvin R. The Measure of Man and Woman: Human Factors in Design. New York: Wiley, 2002.
M U.S. Department of Labor. OSHA Technical Manual, Section VII, Chapter 1, Back Disorders and Injuries. OSHA website: http://www.osha.gov/dts/osta/otm/otm_vii/otm_vii_1.html (visited May 17, 2012).
N U.S. Department of Labor. Hospital eTool: Healthcare Wide Hazards – Ergonomics. OSHA website: http://www.osha.gov/SLTC/etools/hospital/hazards/ergo/ergo.html (visited May 17, 2012).
2.4.3 Impetus for Manufacturers to Improve MDE Accessibility
MDE manufacturers have strong ties to health care professionals and interests in meeting patient care needs. They are committed to making devices that improve and expand the capability of clinical practitioners to provide high quality care. Their design processes are centered around delivering high quality medical devices based on advice primarily from medical professionals, although some manufacturers occasionally seek input from patients during the design phase (e.g., by asking persons with disabilities to test different design options). Manufacturers must follow FDA quality system regulations that require them not only to verify that their devices operate per design but also to validate that the devices meet the needs of health care professionals. MDE manufacturers also are aware of the existing requirements under the ADA and the Rehabilitation Act. Furthermore, highly-publicized legal settlements with medical providers that have required the provision of accessible medical equipment are driving increased demand and an expanding market for accessible MDE.O Equipment designed for accessibility that is currently on the market has satisfied these settlement agreements
Thus, the needs of their institutional and clinical customers strongly influence MDE manufacturers. Health care facilities that would be required to acquire equipment to satisfy new accessibility regulations will quickly look to equipment manufacturers for solutions that are compliant, cost effective, and functional in their workflows and work environments. Being able to fulfill this customer need will thus become both a design input consideration as well as a point of competition among manufacturers.
However, some accessibility design changes will require significant investments to accomplish. For technologies that sell relatively few units annually (e.g., certain highly specialized imaging systems), these design costs may be spread over these few sales, increasing the purchase prices for individual units. For commonly-used items, such as examination tables and chairs, manufacturers could benefit from producing equipment designed for accessibility given the potential for increased demand for these accessible products both in the United States and abroad.
Notes
O Examples of settlements include Metzler v. Kaiser Permanente, Olson v. Sutter Health, University of California San Francisco Medical Center. Full settlement agreements are available online: http://thebarrierfreehealthcareinitiative.org/?page_id=16

User Comments/Questions
Add Comment/Question