Recommendations on Standards for the Design of Medical Diagnostic Equipment for Adults with Disabilities, Advisory Committee Final Report
2. Background
Section 2 describes important contextual considerations that guided the thinking of various Advisory Committee members – from their particular stakeholder perspectives – about the need for and potential nature of accessibility standards for medical diagnostic equipment. These descriptions are not intended as exhaustive or scholarly reviews of particular topics but instead as brief summaries of critical contextual issues that helped shape Advisory Committee members’ views about accessibility standards. Editorial Committee members representing different stakeholder groups prepared the subsections of Section 2 based largely upon their own expertise and experiences.
2.1 Health Care Experiences of Persons with Disabilities
The approximately 57 million U.S. residents living with disabilities vary widely in the nature of the conditions underlying their disabilities and their overall health care needs. On one level, most persons with disabilities require the same services recommended for all individuals to maintain their health and diagnose diseases at early, more treatable stages, such as the screening and preventive services recommended by the U.S. Preventive Services Task Force. A Many persons also require specific diagnostic and therapeutic services because of the health conditions causing their functional impairments and disability. Other persons might need diagnostic testing or therapeutic treatments to address secondary disabilities or conditions related to their primary disabilities. B In addition, as they age, persons with disabilities experience many of the same chronic conditions as do others in late middle-age and older years, such as hypertension, diabetes, cardiovascular and pulmonary diseases, and cancers, necessitating the full range of diagnostic and therapeutic health care services.
Regardless of their health care needs, persons with disabilities are particularly susceptible to experiencing substandard care. Reasons for quality shortfalls run the gamut, from: clinicians’ failures to understand the values, preferences, needs, and expectations of persons with disabilities for their health care (such failures contribute to inadequate or faulty communication, which can compromise care); to financial barriers caused by insufficient or missing health insurance coverage; to inaccessible buildings and medical equipment. In 2000, Healthy People 2010 from the U.S. Department of Health and Human Services, which sets decennial national health priorities, cautioned that "as a potentially underserved group, people with disabilities would be expected to experience disadvantages in health and well-being compared with the general population." 1 The report asserted that common misconceptions about people with disabilities contribute to troubling disparities in the services they receive, especially due to an "underemphasis on health promotion and disease prevention activities."
Numerous other federal reports have highlighted concerns about health care disparities among persons with disabilities. On July 26, 2005, the fifteenth anniversary of the signing of the Americans with Disabilities Act (ADA, P.L. 101-336), the U.S. Surgeon General issued a Call to Action, warning that people with disabilities can lack equal access to health care and urging their inclusion in studies of health care disparities. 2 The National Healthcare Disparities Reports, released annually by the Agency for Healthcare Research and Quality examine disparities in health 3 and dental 4 care for persons with disabilities, among other populations that experience disabilities (e.g., racial and ethnic minorities). C In its 2009 report, the National Council on Disability echoed concerns about health care disparities among persons with disabilities, but underscored the need to gather better data on this issue. 5 The current iteration of Healthy People – Healthy People 2020, the decennial report from the U.S. Department of Health and Human Services that identifies national health improvement priorities for 2010 through 2020 – continues to note health care disparities for persons with disabilities. Among its various objectives for this population, Healthy People 2020 includes decreasing barriers within health care facilities (www.healthypeople.gov/2020).
A growing body of research documents the specific health care disparities experienced by persons with disabilities. Systematically examining this evidence is beyond the scope of this report, but to provide context for later discussions about MDE, we provide one example that exemplifies this research – findings from the National Health Interview Survey (NHIS) D about mammography screening and Pap testing among women with and without disabilities. 6 As shown in Table 2.1, women with self-reported disabilities of different types are substantially less likely than other women to receive these critical screening tests. Among women who self-report mobility difficulties, screening rates fall linearly as the severity of mobility limitations increases. For women reporting the most severe mobility limitations, only 54.9% and 54.2% receive mammography and Pap tests respectively, compared with 74.4% and 82.5% respectively for women reporting no disability.
The NHIS does not ask survey respondents why they do not receive these screening services. Many factors could explain these disparities, including differing health priorities and persons’ preferences for care. E Additional considerations include financial access disparities (e.g., inadequate or absent insurance coverage), transportation problems, and other socioeconomic discrepancies. However, especially for women with mobility disabilities, one explanation is physical barriers to accessing medical equipment, such as examination tables and mammography machines.
Table 2.1
Rates of Mammography and Pap Testing Among
Women with and without Different Disabling Conditions
| Type of difficulty | Mammography in past 2 years* | Pap tests in past 3 years** |
| No disability | 74.4% | 82.5% |
| Movement difficulty (any) | 66.4 | 69.3 |
| Least severe | 75.4 | 79.0 |
| Level 2 | 69.8 | 71.6 |
| Level 3 | 66.3 | 67.9 |
| Level 4 | 59.1 | 60.3 |
| Most severe | 54.9 | 54.2 |
| Seeing or hearing difficulty | 62.8 | 68.8 |
| Emotional difficulty | 58.4 | 72.4 |
| Cognitive difficulty | 52.1 | 58.3 |
*Women age 50 and older **Women age 18 and older
ADAPTED FROM: Altman B, Bernstein A. Disability and Health in the United States, 2001-2005. Hyattsville, MD: National Center for Health Statistics; 2008
The consequences of these health care disparities for persons with disabilities have not yet been fully explored. Healthy People 2010 speculated about the potential for disadvantages in health and well-being for persons with disabilities.1 The possibilities of diagnostic delays and poor patient outcomes from lower use of highly-rated tests like mammography and Pap testing seem clear. More studies are needed to quantify precisely how health care disparities affect the longevity, health, well-being, and quality of life of persons with disabilities.
Notes
A As for persons without disabilities, individual patients with disabilities have their own set of health conditions, including coexisting diseases and health risk factors that might affect the cost-benefit equation of obtaining various health care services. For example, although the U.S. Preventive Services Task Force gives colonoscopy screening to detect colorectal cancers a Grade A (service recommended: high certainty that the net benefit of the service is substantial), individual patients might determine in consultation with their physicians that the clinical risks of the bowel cleansing process required before colonoscopy outweigh the potential benefit of the test in their particular case. This decision should be based on clinical considerations and informed patients’ preferences, not on the inability to accommodate the needs of persons with disabilities during the bowel preparation.
B Secondary disabilities are conditions or complications that are related to a person’s primary disability and are also potentially disabling. Examples include injuries from falls, pressure ulcers, urinary tract complications, and depression.
C Each year, the National Healthcare Disparities Reports look at different measures of disparities, such as different types of service use or different measures of patients’ experiences with care.
D The National Health Interview Survey is a continuous federal survey overseen by the National Center for Health Statistics within the Centers for Disease Control and Prevention (CDC). Over the years, NHIS has been a major source of information about health care disparities for persons with disabilities among other vulnerable populations.
E Although the U.S. Preventive Services Task Force recommends mammography screening (for women ages 50-74 years old) and Pap smears (for women < age 65 who have been sexually active and have a cervix) with Grade B and Grade A evidence, respectively, these recommendations relate to broad populations of women. For each specific woman, the choices about whether to receive these screening services must be assessed based on her individual circumstances. For instance, women with severe, coexisting health conditions and high health risks may decide, in consultation with their physicians, that they will not benefit from this screening and choose not to have the tests.
Section 2: References
1. U.S. Department of Health and Human Services. Healthy People 2010. Second Edition, Understanding and Improving Health and Objectives for Improving Health. Second Edition ed. Washington, D.C.: U.S. Government Printing Office; 2000.
2. U.S. Department of Health and Human Services. The Surgeon General's Call to Action to Improve the Health and Wellness of Persons with Disabilities. Washington, D.C.: Public Health Service, Office of the Surgeon General; 2005.
3. Agency for Healthcare Research and Quality. 2009 National Healthcare Disparities Report. Vol AHRQ Publication No. 10-0004. Rockville, MD: U.S. Department of Health and Human Services; 2010.
4. Agency for Healthcare Research and Quality. 2010 National Healthcare Disparities Report. Vol AHRQ Publication No. 11-0005. Rockville, MD: U.S. Department of Health and Human Services; 2011.
5. National Council on Disability. The Current State of Health Care for People with Disabilities. Washington, DC: National Council on Disability; 2009.
6. Altman B, Bernstein A. Disability and Health in the United States, 2001-2005. Hyattsville, MD: National Center for Health Statistics; 2008.
2.2 Evidence of Physical Accessibility Barriers
A growing number of research publications document physical access barriers involving MDE, including reports concerning: individual patients;7-9 findings from focus groups, in-depth individual interviews, or surveys of relatively small numbers of patients10-21 or practitioners;18, 22 and several larger studies.23-25 One challenge with identifying specific accessibility barriers is the extreme diversity of persons with disabilities and complexity of health care delivery system settings.26, 27 Nonetheless, the group and individual interview studies give voice to the experiences of persons with disabilities, offering insights into their often-shared experiences of physical access barriers involving medical equipment in both diagnosis and treatment settings.7-20
For example, in a study of 20 women with significant mobility difficulties who subsequently developed early-stage breast cancer, women described various strategies for getting onto fixed-height examination tables.11 Several women dismissed as unhelpful step stools or the step built into fixed height tables, including a woman with paraplegia from the long-term effects of childhood polio:
I can’t use the little thing they pull out for you to step up on. No, no, no, that doesn’t work for me. I have to go on the side … in the middle of the table. I belly flop on the table and use my arms to pull me so my body is [lying across the table]. Then I take my arm and lift the leg with the brace … up on the table, and the other one will follow with my body as I try to turn over. Of course, everyone is scared to death that I’m going to fall off the other side. … Mind you, I’m still on my stomach. Now I’m shifting so my head is going towards the top of the table. … Now I’m lengthwise, but I’m on my stomach, so I’ve got to turn over.11
Women reported needing assistance getting onto inaccessible equipment, such as a woman with spinal cord injury who was lifted onto examining tables “by either a couple of nurses or some guys in the hallway.”11 A woman with multiple sclerosis would “usually just ask someone to lift my feet up and to stabilize whatever I’m transferring to if it doesn’t look stable.” Another woman with rheumatoid arthritis said, “I’m afraid of people grabbing me the wrong way. So I have to be careful, and I have to tell them how to handle me.” One woman with cerebral palsy described her staff-assisted transfer onto the examining table as “very awkward and very hard. I had a couple of doctors and nurses. One nurse … strained her back when she was trying to help me get up on the table. I really felt bad about that.”
Most troubling, studies have found that sometimes patients are not transferred onto examination tables for complete physical examinations: instead clinicians examine patients who remain seated in their wheelchairs or scooters. While sometimes this might be reasonable (e.g., if the patient has a condition that does not require complete physical examination), in many situations such limited examinations represent substandard care. For example, in the breast cancer study, physicians often examined women who remained seated in their wheelchairs.11 This represents poor quality care, F as a woman disabled by polio who uses a scooter observed:
Even when I go to my oncologist, he will say, “Oh, don’t bother to get on the table. Just sit in the chair.” Well, I don’t feel I can get an adequate breast examination ... from that particular doctor without being able to ... lay down.11
One woman’s breast surgeon, during their first appointment, said he would examine her in her wheelchair.11 But the woman insisted on being moved to an examining table for a complete evaluation. The surgeon “and this other person lifted me onto the table, but I had to ask to have the breast exam on the table.” These types of stories raise questions about whether diagnoses are delayed by inadequate physical examinations. One woman, who is quadriplegic from polio, reported that her primary care physician always refused to get her out of her wheelchair and instead examined her as she remained seated.14 When she visited a gastroenterologist for inflammatory bowel disease, that physician conducted a complete physical examination with her supine on an exam table. As the woman’s husband observed, the gastroenterologist ‘‘was basically filling in as her primary care.’’ The woman described how her breast cancer was detected: ‘‘He [the gastroenterologist] was examining me, and he went, ‘Uh-oh.’ ‘Uh-oh, what?’ ‘I found a lump.’ So that’s when we found the lump.’’14
No nationally-representative studies have reported on MDE accessibility barriers across the range of health care delivery systems (e.g., private physician offices, health centers, hospital clinics, public health facilities, urgent care centers, practices of other health care professionals such as nurse practitioners, physician assistants, and rehabilitation therapists). Several larger studies give a sense of the prevalence of selected physical access barriers. Although not specific to MDE, one round of the biennial Los Angeles County Health Survey offers prevalence estimates of physical access barriers to health care offices.23 This random-digit-dialed telephone survey of adult (age >18 years), non-institutionalized residents of Los Angeles County occurred from October 2002 through February 2003, with interviews conducted in English, Spanish, and four Asian languages. Of the 14,154 eligible adults contacted, 8,167 (57.7%) completed the telephone interview. Persons who answered “yes” to at least one of three questions about impairments expected to last 3 months were considered disabled.G The survey asked about five barriers to participation in community life, including health care.H Among individuals reporting sensory or physical disabilities, 22.0% indicated difficulty accessing offices of health care providers because of its physical layout or location. Among non-Hispanic respondents, blacks were significantly more likely than whites to report access barriers (33.0 versus 14.4%). Persons with the most severe disabilities reported significantly more difficulties than did persons with the least severe disabilities (30.9 versus 13.8%). Commenting on these results, which appeared in their publication, the editors of Morbidity and Mortality Weekly Report from the CDC noted, “Accessibility to offices of healthcare providers could be improved by lowering service counters and examination tables and ensuring that scales are wheelchair accessible.”23
Another report from California provides perhaps the most specific information about the prevalence of physical access barriers, including MDE. Mudrick and colleagues analyzed findings from a 55-item instrument that assessed medical office or clinic parking, exterior access, building entrances, interior public spaces, doctor's office interiors, and the presence of accessible examination equipment.24 Using this instrument, 5 health plans serving California Medicaid patients conducted reviews of providers that had signed with their plans. These data were merged across plans for analysis. With the exception of van accessible parking (which was inadequate), parking, exterior access, building access, and interior public spaces generally complied with the access criteria. However, barriers were found frequently in bathrooms and examination rooms. In particular, only 3.6% of the sites had an accessible weight scale, and just 8.4% had a height adjustable examination table.24
To learn about the accessibility of medical and surgical subspecialist practices for patients who use wheelchairs, Lagu and colleagues conducted a “secret shopper”-type telephone survey.25 The researchers called subspecialty offices ostensibly to make an appointment for a fictional patient with hemiparesis who was obese, used a wheelchair, and could not self-transfer from the wheelchair chair to an examination table. They spoke with 256 endocrinology, gynecology, orthopedic surgery, rheumatology, urology, ophthalmology, otolaryngology, and psychiatry practices in 4 U.S. cities and asked about the accessibility of the practice, reasons for lack of accessibility, and the planned method of transfer of the patient to an examination table. Of the 256 practices, 56 (22%) reported that they could not accommodate the patient; 9 (4%) indicated that their building was inaccessible; and 47 (18%) said they could not transfer the patient from the wheelchair to an examination table. Only 22 (9%) reported use of either a height-adjustable examination table or a lift for transfer. Among the various specialties, gynecology had the highest rate of inaccessible practices (44%).25
Notes
F The clinical breast exam requires clinicians to palpate the entire breast, its perimeter, and immediately adjacent areas including the axilla (e.g., checking for lymph nodes). The breast tissue must be spread evenly over the chest wall, which requires women to be supine. With the woman lying flat on her back on an examination table, positioning her arm toward her head and rotating her hip and torso can assist in spreading the breast tissue.
G (1) “Are you limited in any way in any activities because of a physical, mental, or emotional problem?” (2) “Do you now have any health problems that require you to use special equipment such as a cane, a wheelchair, a special bed, or a special telephone?” and (3) “Do you consider yourself a person with a disability?”23 Those classified as having a disability were then asked about whether their disability was physical, sensory, mental, or learning (they could report more than one type) and whether their disability was slight, moderate, or severe (based on their own perceptions).
H (1) Experiencing restricted social activity. (2) Not knowing where to obtain disability resource information. (3) Needing home modifications but not having them. (4) Having difficulty accessing a health care provider’s office because of its physical layout or location. (5) being treated unfairly at a health care provider’s office.23
Section 2: References
7. Andriacchi R. Primary care for persons with disabilities. the internal medicine perspective. Am J Phys Med Rehabil. 1997;76(3 Suppl):S17-20.
8. Iezzoni LI. Blocked. Health Aff (Millwood). 2008;27(1):203-209. doi: 10.1377/hlthaff.27.1.203.
9. Kirschner KL, Breslin ML, Iezzoni LI. Structural impairments that limit access to health care for patients with disabilities. JAMA. 2007;297(10):1121-1125.
10. Drainoni M, Lee-Hood E, Tobias C, Bachman SS, Andrew J, Maisels L. Cross-disability experience of barriers to health-care access: Consumer perspectives. Journal of Disability Policy Studies. 2006;17(2):101-115.
11. Iezzoni LI, Kilbridge K, Park ER. Physical access barriers to care for diagnosis and treatment of breast cancer among women with mobility impairments. Oncology Nursing Forum. 2010;37(6):711-717.
12. Iezzoni LI, Killeen MB, O'Day BL. Rural residents with disabilities confront substantial barriers to obtaining primary care. Health Serv Res. 2006;41(4 Pt 1):1258-1275.
13. Iezzoni LI, O'Day BL. More than Ramps. A Guide to Improving Health Care Quality and Access for People with Disabilities. New York: Oxford University Press; 2006.
14. Iezzoni LI, Park ER, Kilbridge K. Implications of mobility impairment on the diagnosis and treatment of breast cancer. Journal of Women's Health. 2011;20(1):45-52.
15. Kroll T, Jones GC, Kehn M, Neri MT. Barriers and strategies affecting the utilisation of primary preventive services for people with physical disabilities: A qualitative inquiry. Health Soc Care Community. 2006;14(4):284-293.
16. Lishner DM, Richardson M, Levine P, Patrick D. Access to primary health care among persons with disabilities in rural areas: A summary of the literature. J Rural Health. 1996;12(1):45-53.
17. Mele N, Archer J, Pusch BD. Access to breast cancer screening services for women with disabilities. J Obstet Gynecol Neonatal Nurs. 2005;34(4):453-464.
18. Morrison EH, George V, Mosqueda L. Primary care for adults with physical disabilities: Perceptions from consumer and provider focus groups. Fam Med. 2008;40(9):645-651.
19. Scheer JM, Kroll T, Neri MT, Beatty P. Access barriers for persons with disabilities: The consumer's perspective. J Disabil Policy Stud. 2003;14(4):221-230.
20. Smeltzer SC, Sharts-Hopko NC, Ott BB, Zimmerman V, Duffin J. Perspectives of women with disabilities on reaching those who are hard to reach. J Neurosci Nurs. 2007;39(3):163-171.
21. Story MF, Schwier E, Kailes JI. Perspectives of patients with disabilities on the accessibility of medical equipment: Examination tables, imaging equipment, medical chairs, and weight scales. Disabil Health J. 2009;2(4):169-179.e1.
22. Bachman SS, Vedrani M, Drainoni M, Tobias C, Maisels L. Provider perceptions of their capacity to offer accessible health care for people with disabilities. J Disabil Policy Stud. 2006;17(3):130-136.
23. Centers for Disease Control and Prevention. Environmental barriers to health care among persons with disabilities, Los Angeles county, California, 2002-2003. Morbidity and Mortality Weekly Report. 2006;55(48):1300-1303.
24. Mudrick NR, Breslin ML, Liang M, Yee S. Physical accessibility in primary health care settings: Results from california on-site reviews. Disabil Health J. 2012;5(3):159-167.
25. Lagu T, Hannon NS, Rothberg MB, et al. Access to subspecialty care for patients with mobility impairment: A survey. Ann Intern Med. 2013;158(6):441-446.
26. Story MF, Winters JM, Lemke MR, et al. Development of a method for evaluating accessibility of medical equipment for patients with disabilities. Appl Ergon. 2010;42(1):178-183.
27. Story MF, Kaile JI, MacDonald C. The ADA in action at health care facilities. Disabil Health J. 2010;3:245-252.
2.3 Implications of MDE Accessibility for Clinical Staff
Health care personnel face risks of injuries from transferring, positioning, or otherwise physically assisting patients in health care settings. Surgeon Pauline W. Chen, MD, described a failed attempt to transfer a patient onto a fixed height table, capturing the consequences both for the patient and what became the transfer crew – a physician, medical student, several clinical staff, and two security guards (Box 2.3).
Box 2.3
In his 60s, overweight and in a wheelchair, the patient had been seeing doctors and nurses regularly for his diabetes. Only recently had they discovered a pressure sore after someone had finally, as he put it, “wanted to examine at my backside.”
The oversight struck me as unimaginable. Until I watched another doctor try.
My colleague, a strapping man in his 30s, wrapped his arms around the man’s torso to lift him onto the examining table but could hardly budge the patient. A few members of the clinic staff came in to help, each taking a limb. Several minutes later, one of the nurses called for security. Two burly men in dark blue uniforms joined the fray, grunting as they finally extricated the patient from his chair.
A nurse lunged forward to unbuckle the patient’s belt while a medical student began yanking on his sneakers, but with each tug and jerk, the guards’ grip on the patient’s torso loosened. Feeling himself slipping, the patient grabbed at the shirt of one of the guards to break his fall. The guard lost his balance and reached for the wheelchair, but its brake was not engaged. The wheelchair spun, hitting the medical student and nurse and knocking over the other guard as the patient, pants half off and one shoe missing, collapsed back into its seat.
No one was hurt. But when my colleague leaned down to ask the patient how he was, he stopped himself midquestion. Though the patient’s black baseball cap now partly obscured his face, it was clear to all of us what his expression conveyed: utter humiliation.
SOURCE: Chen PW, “Disability and Discrimination at the Doctor’s Office,” New York Times, Doctor and Patient, Health Blogs, May 23, 2013
http://well.blogs.nytimes.com/2013/05/23/disability-and-discrimination-at-the-doctors-office/?hpw
Thousands of health care personnel are injured every year from manually lifting patients, including persons with and without disabilities. Direct-care registered nurses rank tenth among all occupations for developing musculoskeletal disorders.28 Because of back injuries from manually moving patients, 12% of nurses leave the profession every year.29 In 2010, nursing aides, orderlies, and attendants experienced:30
-
An incident rate of 249 cases/10,000 full-time workers for musculoskeletal disorder (MSD) cases with days away from work;
-
27,020 MSD cases with days away from work; and
-
An incident rate of 283 cases/10,000 full-time workers for nonfatal occupational injuries and illnesses involving days away from work.
This high incidence of injuries among clinical staff has heightened attention by government, health care professional, and industry leaders. In 2009, federal legislation was introduced to address this problem: The Nurse and Healthcare Worker Protection Act (H.R 2381). While this legislative effort failed, it is likely that similar legislation will be proposed in future Congressional sessions. Currently, “Safe Patient Handling” (SPH) laws have been enacted in 10 states,31 and Hawaii has passed a SPH resolution. As of June 2013, the American Nurses Association had released National Practice Standards for Safe Patient Handling.32
The National Institute for Occupational Safety and Health (NIOSH), in the CDC, recommends manual lifting of no more than 51 pounds in ideal conditions. In 1994, NIOSH/CDC) released the Application Manual for the Revised NIOSH Lifting Equation,33 which provides an ergonomics assessment tool for calculating the recommended weight limit for two-handed manual-lifting tasks. However, NIOSH excluded assessment of patient-handling tasks from the uses of the revised equation, arguing that such tasks involve too many variables. Nevertheless, the NIOSH Lifting Equation can be used to calculate a recommended weight limit for a limited range of patient-handling tasks in which the patient is cooperative and unlikely to move suddenly during the task. In general, the revised equation yields a recommended 35-pound maximum weight limit for these patient-handling tasks. When the weight to be lifted exceeds 35 pounds, assistive devices should be used.34
As suggested above,25 the availability of these assistive devices is still quite limited especially in doctors’ offices, clinics, and specialists’ offices where many examinations and medical diagnostic tests occur. When assistive devices are not available, clinical staff must manually transfer patients to exam tables and onto diagnostic equipment. Such transfers greatly exceed the 35-lb maximum weight limit and place health care workers at significant risk of injury. However, if equipment is designed such that patients in wheelchairs and scooters can transfer independently or with moderate assistance, the risk of injury to health care workers can be greatly reduced for this task. The use of lifts also can reduce the risk of injury for patients and staff. The risk of injury will also decrease with improved accessibility of medical diagnostic equipment for other individuals with limited mobility, such as the older individuals, pregnant women, and persons with extreme obesity.
Section 2: References
25. Lagu T, Hannon NS, Rothberg MB, et al. Access to subspecialty care for patients with mobility impairment: A survey. Ann Intern Med. 2013;158(6):441-446.
25. Lagu T, Hannon NS, Rothberg MB, et al. Access to subspecialty care for patients with mobility impairment: A survey. Ann Intern Med. 2013;158(6):441-446.
28. Representative John Conyers Jr. (D-MI14). Nurse and health care worker protection act of 2009. 2009;H.R. 2381 (111th).
29. American Nurses Association. AMA leads initiative to develop national safe patient handling standards. multidisciplinary group seeks to establish evidence based guidelines to address deficiency. http://nursingworld.org/MainMenuCategories/WorkplaceSafety/Healthy-Work-Environment/SafePatient/ANA-Leads-National-Safe-Patient-Handling-Standards.pdf. Accessed May 31, 2013.
30. Bureau of Labor Statistics, U.S. Department of Labor. News release: Nonfatal occupational injuries and illnesses requiring days away from work, 2011. November 8, 2012;USDL-12-2204.
31. American Nurses Association, Nursing World. Saftey patient handling and mobility, health and safety. http://nursingworld.org/handlewithcare. Accessed April 27, 2012.
32. American Nurses Association, Nursing World. Safe patient handling and mobility (SPHM), state legislative agenda. http://nursingworld.org/MainMenuCategories/Policy-Advocacy/State/Legislative-Agenda-Reports/State-SafePatientHandling. Accessed April 27, 2012.
33. Waters TR, Putz-Anderson V, Garg A. Application Manual for the Revised NIOSH Lifting Equation. Pub. No. 94-110 ed. Cincinnati, OH: Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Division of Biomedical and Behavioral Science, DHHS (NIOSH); 1994.
34. Waters TR. When is it safe to manually life a patient?. AJN. 2007;107(8):53-58.
2.4 MDE Manufacturers and Accessibility
2.4.1 Current Marketplace Dynamics
The Americans with Disabilities Act and Section 504 of the Rehabilitation Act of 1973 already require that covered medical care providers ensure the provision of medical care and services to persons with disabilities in a nondiscriminatory manner. These federal laws also require that persons with disabilities have an equal opportunity to participate in and benefit from the providers’ medical services, including having access to health care facilities and to the medical equipment used to provide services. Accessible medical equipment is required in order to meet this nondiscrimination obligation and eliminate the barriers that inaccessible equipment can create for persons with disabilities. As is discussed below, the regulations implementing these federal laws do not currently include specific technical requirements for the accessibility of non-fixed medical equipment, although steps are underway by the Department of Justice to propose specific ADA technical standards for medical equipment.
Even without specific technical requirements for non-fixed medical equipment, the examination table marketplace is developing and selling more equipment intended to meet accessibility needs. As noted above, historically, fixed-height examination tables set at 32” for the convenience of clinicians have dominated the health care delivery system. Increasingly, these so-called “box tables” are being replaced by examination tables that are height adjustable, such as that shown in Figure 2.4.1(a). Typically, height adjustable tables today lower to about 19” from the floor.I Sales figures from examination table manufacturers indicate that in 2012, about 25% of examination tables sold are height-adjustable (Figure 2.4.1(b)).
Other MDE manufacturers have also begun addressing accessibility issues, primarily within certain specialized populations. For example, manufacturers of diagnostic imaging equipment have produced equipment specifically to serve pediatric populations and persons with extreme obesity (so-called “bariatric” equipment).J Diagnostic imaging equipment manufacturers are also designing equipment for specific health care delivery settings, including facilities with lower financial resources (e.g., institutions in rural regions, developing countries) and hospital emergency departments. As part of designing for broader patient populations and delivery settings, new equipment designs incorporate patient and clinician usability considerations, such as adjustable table heights and table minimum heights.
Figure 2.4.1(a)
Height Adjustable and Fixed-Height “Box” Examination Tables
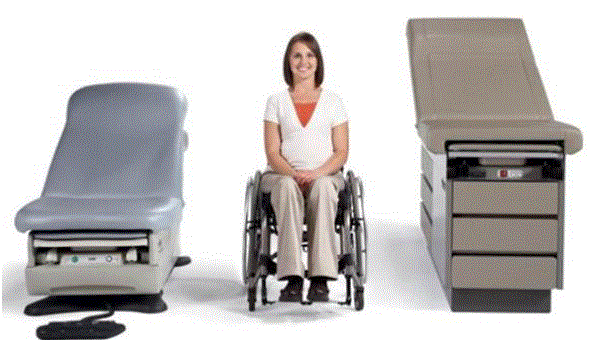
(SOURCE: Midmark Corporation) Fixed height table on right, adjustable height table on left.
Figure 2.4.1(b)
Types of Examination Tables Sold: 2005-2012
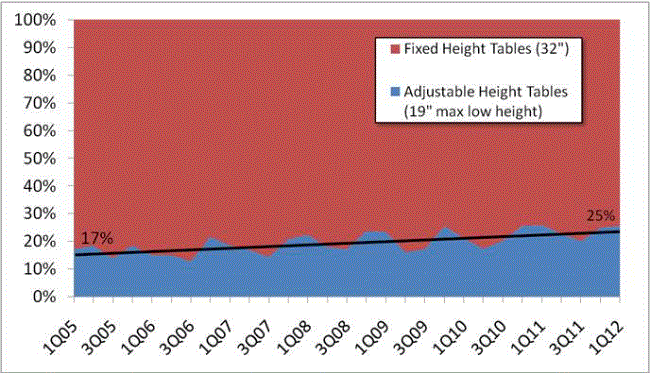
(SOURCE: Global Healthcare Exchange (GHX)) The y axis indicates the percent of tables sold that are adjustable height (blue) and fixed height (red). The x axis indicates the quarter and year of the data (e.g., first quarter in 2005, extreme left).
Notes
I The methods for measuring “low heights” for current height adjustable products vary among manufacturers and product designs. No official measurement method currently exists. Sections 4.1.2 and 5.1.4 describe the new standardized measurement method proposed by the Advisory Committee. Heights referenced in this report utilize this proposed new measurement method.
J As noted in Sections 1 and 8, this report does not address accessibility standards for children or for individuals with extreme obesity.
2.4.2 Considerations of Manufacturers in Accessible MDE Design
MDE plays a central role in ensuring the health and well-being of all individuals by supporting the detection of diseases and disorders – essential information for developing treatments or therapeutic regimens to cure, control, or significantly palliate a wide range of health problems across the life span. Therefore, an immutable core attribute of MDE must be its ability to effectively support accurate and timely diagnoses. Other key factors that guide MDE manufacturers in their equipment design include: the nature of the specific diagnostic objective; safety requirements; FDA regulations (specifically 21CFR Part 820 – Quality Systems Regulations) and processes (Section 2.5); expected patient and user demographics; international standards for safety, essential performance, and usability; ergonomic guidelines; and ultimately, validation with representative customers to help establish the safety and effectiveness of the medical devices. MDE manufacturers believe that their usability requirements should include some measure of accessibility.
As detailed in Section 2.5.2.1, the most common standard used by manufacturers to guide the design of medical equipment is ANSI/AAMI ES60601-1:2005K, which is the U.S. version of the larger scope of the International Electrotechnical Commission’s IEC 60601 series of standards for basic safety and essential performance of medical electrical equipment which also must be met. Imaging equipment that utilizes ionizing radiation must also comply with 21CFR Subchaper J. As detailed further in Section 2.5, all medical devices must adhere to FDA quality system regulation (21CFR820), applicable pre-market notification or approval processes, and risk management performed in accordance with ISO 14971.
These standards, regulations, and recommended practices provide details concerning the elements supporting the basic safety and essential performance of MDE. Standards with particular relevance to accessibility considerations include:
-
Instability hazards: including the risk of the equipment tipping. Avoiding dangerous tipping is particularly important as patients transfer onto or off of MDE or reposition themselves upon it. As the width of MDE, such as examination tables and chairs increases, equipment may require redesign to address changes in tipping hazards. To mitigate tipping risks, the size of table and chair bases can be increased. This increase in base size can affect lift capability, reduce the open areas around equipment in the examination room, and require significant product redesigns (e.g., to ensure lift compatibility).
-
Patient support safety factors: Patient support devices must meet applicable safety factors as delineated in IEC 60601-1. These factors typically range from 4x to 8x. This means a patient table labeled to support a 500 lb. patient must actually be designed and tested at up to 4,000 lbs. This has significant implications for adjustable height table design as many designs lose mechanical advantages as they go lower.
To guide their design efforts, manufacturers rely on anthropometric information,L such as the specific measurements of men and women from the 5th to 95th percentiles. They also must consider the use of the MDE for the operator, including accessibility for the operator, topics which are not addressed in the NPRM. For example, accessibility standards could define operator requirements for communication (M306) and operable parts (M307). This is particularly important regarding standards for proper ergonomics for lifting and bending, which should meet Occupational Safety and Health Administration (OSHA) Standards (Section 2.3).M
For clinical personnel, as the size of the transfer surface increases, the likelihood rises of putting clinical personnel into an unfavorable ergonomic position as a result of increasing their need to reach, lean or stretch. OSHA standards speak directly to this issue of ergonomic hazards within health care, including specific concerns about awkward postures and patient handling.N
Another critical factor that must be considered is the ability of clinical staff to access the patient during a diagnostic imaging exam to ensure proper positioning, administer imaging agents or other drugs, monitor the patient, respond to patients’ requests, and so on. Additionally, because in most cases, the tables on diagnostic imaging equipment move during the exam; tables may have both moveable and stationary parts. Therefore, design consideration must be given to avoid tubing and other such items that are attached to the patient from getting caught in a tableside support and pulled out of a patient with the potential for injuring patients, compromising image quality, or necessitating another imaging procedure.
Notes
K Association for the Advancement of Medical Instrumentation. ANSI/AAMI ES60601-1:2005 (R) 2012. Medical Electrical Equipment—Part 1: General Requirements for Basic Safety and Essential Performance. Approved 9 February 2006 by American National Standards Institute, Inc. Revised 2012.
L Tilley, Alvin R. The Measure of Man and Woman: Human Factors in Design. New York: Wiley, 2002.
M U.S. Department of Labor. OSHA Technical Manual, Section VII, Chapter 1, Back Disorders and Injuries. OSHA website: http://www.osha.gov/dts/osta/otm/otm_vii/otm_vii_1.html (visited May 17, 2012).
N U.S. Department of Labor. Hospital eTool: Healthcare Wide Hazards – Ergonomics. OSHA website: http://www.osha.gov/SLTC/etools/hospital/hazards/ergo/ergo.html (visited May 17, 2012).
2.4.3 Impetus for Manufacturers to Improve MDE Accessibility
MDE manufacturers have strong ties to health care professionals and interests in meeting patient care needs. They are committed to making devices that improve and expand the capability of clinical practitioners to provide high quality care. Their design processes are centered around delivering high quality medical devices based on advice primarily from medical professionals, although some manufacturers occasionally seek input from patients during the design phase (e.g., by asking persons with disabilities to test different design options). Manufacturers must follow FDA quality system regulations that require them not only to verify that their devices operate per design but also to validate that the devices meet the needs of health care professionals. MDE manufacturers also are aware of the existing requirements under the ADA and the Rehabilitation Act. Furthermore, highly-publicized legal settlements with medical providers that have required the provision of accessible medical equipment are driving increased demand and an expanding market for accessible MDE.O Equipment designed for accessibility that is currently on the market has satisfied these settlement agreements
Thus, the needs of their institutional and clinical customers strongly influence MDE manufacturers. Health care facilities that would be required to acquire equipment to satisfy new accessibility regulations will quickly look to equipment manufacturers for solutions that are compliant, cost effective, and functional in their workflows and work environments. Being able to fulfill this customer need will thus become both a design input consideration as well as a point of competition among manufacturers.
However, some accessibility design changes will require significant investments to accomplish. For technologies that sell relatively few units annually (e.g., certain highly specialized imaging systems), these design costs may be spread over these few sales, increasing the purchase prices for individual units. For commonly-used items, such as examination tables and chairs, manufacturers could benefit from producing equipment designed for accessibility given the potential for increased demand for these accessible products both in the United States and abroad.
Notes
O Examples of settlements include Metzler v. Kaiser Permanente, Olson v. Sutter Health, University of California San Francisco Medical Center. Full settlement agreements are available online: http://thebarrierfreehealthcareinitiative.org/?page_id=16
2.5 National and International Standards Settings Organizations and MDE Activities
As mentioned in Section 2.4.2, MDE must already meet a variety of standards before it can be marketed in the U.S. This section gives an overview of these standards, starting with medical device provisions of the FDA.
2.5.1 U.S. Food and Drug Administration
FDA is responsible for ensuring that medical devices that manufacturers want to introduce into commerce in the U.S. are safe and effective for their intended uses and user populations.
FDA’s Quality System Regulation was codified in Title 21, Part 820 of the Code of Federal Regulations (21 CFR 820), which incorporates current good manufacturing practice (cGMP) requirements. The regulation requires manufacturers to follow a rigorous and multifaceted process for design, manufacturing, post-market review and adverse event reporting of medical devices and accessories. These include implementation of design controls, document controls, purchasing controls, product identification and traceability, production and process controls, criteria for acceptance and non-conformance of products, corrective and preventive actions, labeling (including user information, marketing claims, and product promotion), storage/distribution/installation, record keeping, and servicing. These requirements mean that few changes to medical devices can be treated as “small” and rigorous processes must be followed to ensure the product continues to perform as designed and intended in a reliable and safe fashion.
FDA classifies medical devices based on the level of risk of harm they present to patients and users. There are three classes, ordered from low to high risk: Class I, II, and III. With few exceptions, the medical diagnostic equipment covered by the proposed accessibility standards is either FDA Class I or Class II.
-
Class I devices typically do not require any form of premarket clearance or approval from the FDA, but the manufacturers are still required to follow the FDA Quality System Regulation (in 21 CFR 820) and are subject to FDA audit. Examples include examination tables and chairs.
-
Class II devices are required to follow the FDA Quality System Regulation and usually must receive FDA clearance prior to being introduced into commerce in the US. To receive FDA clearance, the manufacturer must submit a premarket notification (known as a “510(k)” per 21 CFR 807, Subpart E) if a device is being introduced into commercial distribution for the first time, or if a device has been changed in a way that could significantly affect the device’s safety or effectiveness. The FDA performs scientific and clinical reviews of the contents of 510(k) submissions to determine whether the device is substantially equivalent to a predicate device already in commercial distribution. Examples include most diagnostic imaging systems.
-
Class III devices either represent new device technologies or are devices (such as life supporting devices) that are associated with the highest levels of risk because they have the greatest potential for adverse public health effects if problems, such as device malfunctions or use errors, occur. This class of devices must go through a more rigorous FDA premarket process, specified in 21 CFR 814, for premarket approval (PMA). Currently only some digital mammography devices covered under the proposed accessibility standards fall into this classification.
FDA would become involved in implementation of the proposed accessibility standards when a manufacturer wants to introduce into commercial distribution in the U.S. a new medical device or device accessory that complies with the standards or to modify an existing device to comply with the standards (Section 7.4 describes this process in greater detail). All devices that fall into Class II or Class III are subject to the FDA premarket clearance or approval process. FDA reviews the premarket submission to confirm that the device is safe and effective for its intended uses and user population. In addition, a manufacturer stating in its labeling that the device conforms to the new standards would be making a marketing claim that FDA would then be required to review and verify was accurate. A manufacturer who could comply only partially with the new standards could identify the aspects of the device that meet the requirements in the standards and, therefore, meet the criteria for FDA to allow them to be described in the device labeling as “accessible.” However, any final determinations of accessibility would be made by the Department of Justice, which will be responsible for issuing any enforceable medical equipment standards. Such determinations will stem not only from the design of the medical equipment but also other concerns, such as the placement of the equipment in examination rooms.
2.5.2 Major Relevant U.S. and International Standards
2.5.2.1 International Electrotechnical Commission (IEC) Standards
IEC standards are written by experts nominated by their country’s national committees. Representatives of manufacturers, regulatory agencies (including FDA), and user groups (e.g., radiologists, physicians from other clinical specialties, physicists, technologists) constitute the groups of experts. The draft standards are reviewed globally and voted on by each national committee.
The primary standards used in the design of the MDE covered by the recommended disability access standards is the IEC 60601-1 series of standards for basic safety and essential performance of medical electrical equipment. Most MDE covered by new accessibility standards would fall into the scope of IEC 60601-1 series, a tiered set of standards that is globally recognized. It is part of the regulatory approval process for applicable medical equipment in the vast majority of countries that have a formalized regulatory approval/registration process. The U.S. developed a companion standard to IEC 60601-1, designated ANSI/AAMI ES60601-1, which contains national deviations from the general standard and country-specific requirements (i.e., additions) to the standard.
The base standard, referred to as the “general standard” in this tiered system, is IEC 60601-1, “Medical electrical equipment – Part 1: General requirements for basic safety and essential performance.” The standard focuses on electrical, mechanical, and radiation safety, as well as hazards related to temperature extremes (e.g., fire), control accuracy, fault conditions, software design, construction, and compatibility with other systems. It also looks at what is considered essential performance for the equipment.
The general standard, IEC 60601-1, is complemented by a set of “collateral” standards, designated as the IEC 60601-1-x series of standards. These collateral standards can add, subtract, and/or modify the requirements of the general standard for the specific systems and topics to which they apply. For example, IEC 60601-1-3, “Radiation protection in diagnostic X-ray equipment,” addresses the requirements for radiation safety for all X-ray equipment.
Another example of the collateral standards is IEC 60601-1-6, “Usability,” which applies to all equipment covered by the general standard. However, it maps directly to IEC 62366, “Medical devices – Application of usability engineering to medical devices,” and varies little except to identify slight differences in terminology between the two documents. Both 60601-1-6 and 62366 are process standards that describe a process for evaluating the usability (related to safety) of medical devices. These standards are becoming more widely recognized and followed.
IEC 60601-1 is also accompanied by a set of “Part 2” or “Particular” standards, designated as the 60601-2-xx series of standards. These particular standards are written specifically to address the unique aspects of one particular type of medical electric equipment. They can add, subtract, and/or modify the requirements of either the general standard or any collateral standard for the equipment to which it applies. For example, IEC 60601-2-45 applies to mammography equipment, and IEC 60601-2-52 applies to medical beds, including stretchers. Part 2 standards do not exist for all medical equipment types; for example, no Part 2 standard is specified for examination tables and chairs. When there is no Part 2 standard, equipment is typically tested only to the general standard (i.e., IEC 60601-1).
Conformance to these series of standards is accessed via testing by an Occupational Safety and Health Administration (OSHA) certified Nationally Recognized Testing Laboratory (NRTL) such as Underwriters Laboratories (UL). The NRTL authorizes the manufacturer to apply the test house’s symbol to the medical device. This certification is required by electrical inspectors prior to introducing a piece of medical electrical equipment into a medical facility. In addition, as part of its premarket notification (510(k)) clearance process and its premarket approval process (PMA), the FDA requires manufacturers to submit evidence of conformance to these standards as part of the information they provide to demonstrate that the device is safe and effective.
2.5.2.2 International Organization for Standardization (ISO) Standards
The primary standard from ISO used in the design of medical devices is ISO 14971, “Medical devices - Application of risk management to medical devices.” Risk management plays a critical and central role in the design of medical devices, and it also ties into IEC 60601-1 and FDA’s expectations for quality systems and premarket clearance or approval. Potential hazards must be identified and then assigned ratings of the probability of occurrence of harm and of the severity of the consequences of that harm. These ratings are combined to generate a risk level. If the risk level is high enough, the design must incorporate mitigations to control the risk. The risk mitigation measures must also be assessed to confirm their effectiveness at reducing the risks to acceptable levels and to ensure that they did not introduce any new hazards or risks.
2.5.2.3 FDA Standards
The FDA maintains a list of recognized consensus standards on their web site,P which includes standards from IEC, ISO, and other organizations. Manufacturers and assemblers of systems use these standards to demonstrate that a product is safe and effective. Depending on the classification of the equipment (see Section 2.5.1), supporting evidence of compliance to the applicable performance standards may need to be submitted to the FDA for review and approval.
Notably, among the FDA’s recognized consensus standards is ANSI/AAMI HE 75,Q recommended practices for human factors design principles for medical devices. Chapter 16 of ANSI/AAMI HE 75 contains recommended practices regarding accessibility for patients and health care personnel with disabilities.
For radiation emitting devices (such as some diagnostic imaging equipment), FDA has a set of performance standards that focus on radiation safety to the operator and patient. These are found in the Code of Federal Regulations, specifically in Title 21, Part 1020 (21 CFR 1020), Performance Standards for Ionizing Radiation-Emitting Products, and Part 1050 (21 CFR 1050), Performance Standards for Sonic, Infrasonic and Ultrasonic Radiation-Emitting Products. Manufacturers and assemblers of applicable systems must file a report to FDA that includes test data that demonstrates compliance to the applicable performance standards for the device in question.
Notes
P This list of consensus standards recognized by the FDA is available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfstandards/search.cfm
Q Information about the human factors design principles for medical devices is available at: http://www.aami.org/he75
2.6 Brief History of Section 4203 of the Patient Protection and Affordable Care Act
The Section 4203 provisions of the Patient Protection and Affordable Care Act (ACA), which required this accessibility standard setting, apply exclusively to medical diagnostic equipment and thus leave unaddressed equipment used only for therapeutic purposes. Therefore, to set the full context for the MDE Advisory Committee’s role and the recommended accessibility standards, this section briefly reviews the origins of what became Section 4203.
In 2002, the National Institute on Disability and Rehabilitation Research (NIDRR) in the U.S. Department of Education awarded a five-year grant to Marquette University and its collaboratorsR to form the Rehabilitation Engineering Research Center (RERC) on Accessible Medical Instrumentation. One of the major projects funded by the grant was a study about the types of medical equipment that were most difficult for patients with disabilities to use and what caused any use difficulties discovered. The project used research methods that included (in chronological order) a national online survey, a series of regional focus groups, and a set of targeted usability studies.21, 35, 36
The national survey results showed that the following five types of medical equipment were moderately difficult, extremely difficult, or impossibly difficult to use by ≥ 50% of the respondents:
-
Exam tables (n = 291) 75.0%
-
X-ray equipment (n = 258) 68.0%
-
Rehabilitation/exercise equipment (n = 203) 55.3%
-
Weight scales (n = 222) 53.1%
-
Exam chairs (n=262) 49.6%
The project staff found that, with the exception of rehabilitation/exercise equipment, the other four most-reported categories of inaccessible medical equipment were exam tables, x-ray equipment, weight scales, and exam chairs – which are all essential for basic health care.
The researchers investigated the details of use difficulties through a series of regional focus groups with individuals who had a variety of disabilities and then conducted a set of targeted usability studies of the most inaccessible types of medical equipment to observe and characterize the sources of the difficulties. They also participated with the Association for the Advancement of Medical Instrumentation’s Human Factors Engineering Committee (AAMI/HE) to include a chapter on Accessibility Considerations into its standard, HE75:2009, “Human factors engineering – Design of Medical Devices.” The chapter provided recommendations for the types of medical equipment named above to increase its accessibility for patients with disabilities.
In 2007, as the RERC-AMI was finishing its projects, members of the U.S. Senate and the House of Representatives introduced legislation to address the problem. In the Senate, Senator Tom Harkin (D-IA) introduced S. 1050 and in the House, Representative Nita Lowey (D-NY) introduced the companion bill, H.R. 3294. Titled the “Promoting Wellness for Individuals with Disabilities Act,” the bills sought to establish standards for basic medical equipment, among other provisions. These bills were later incorporated into the ACA as Section 4203, “Removing Barriers and Improving Access to Wellness for Individuals with Disabilities.” As described in Section 1.1, this ACA provision adds to Title V of the Rehabilitation Act of 1973 a new Section 510, “Establishment of standards for accessible medical diagnostic equipment.” The law requires the US Access Board, in consultation with the U.S. Food and Drug Administration (FDA), to develop such standards, which are to apply specifically to examination tables, examination chairs (e.g., for eye or dental examinations and procedures), weight scales and imaging equipment (e.g., mammography equipment, x-ray machines).
Notes
R Western University of Health Sciences, University of California - San Francisco/Berkeley, University of Connecticut, University of Wisconsin-Milwaukee
Section 2: References
21. Story MF, Schwier E, Kailes JI. Perspectives of patients with disabilities on the accessibility of medical equipment: Examination tables, imaging equipment, medical chairs, and weight scales. Disabil Health J. 2009;2(4):169-179.e1.
35. Story MF, Winters JM, Premo B, Kailes JI, Schwier E, Winters JM. Focus Groups on Accessibility of Medical Instrumentation. Atlanta, GA: Proceedings of RESNA 2005 Conference; 2005.
36. Winters JM, Story MF, Barnekow K, et al. Accessiblity of Medical Instrumentation: A Natilonal Healthcare Consumer Survey. Atlanta, GA: Proceedings of RESNA 2005 Conference; 2005.
2.7 Existing ADA and Rehabilitation Act Requirements for Accessible Medical Care
The Americans with Disabilities Act (ADA) and Section 504 of the Rehabilitation Act are civil rights laws that prohibit discrimination on the basis of disability. Title II of the ADA (42 U.S.C. 12131 to 12165) applies to state and local governments, and Title III of the ADA (42 U.S.C. 12189 to 12189) applies to private entities that are public accommodations such as health care providers. Section 504 of the Rehabilitation Act (29 U.S.C. 794) applies to recipients of federal financial assistance, such as Medicare, Medicaid, and other federal programs. ADA and Section 504 of the Rehabilitation Act require health care practitioners and delivery systems to provide individuals with disabilities full and equal access to their health care services and facilities. DOJ has entered into settlement agreements with health care providers to enforce these requirements. The settlement agreements require the health care providers to acquire accessible medical equipment.
In July 2010, DOJ and the Department of Health and Human Services issued a guidance document for health care providers regarding their responsibilities to make their services and facilities accessible to individuals with mobility disabilities under the ADA and Section 504 of the Rehabilitation Act (see Access to Medical Care for Individuals with Mobility Disabilities available at: http://www.ada.gov/medcare_ta.htm). The guidance document includes information on accessible examination rooms and the clear floor space needed adjacent to medical equipment for individuals who use mobility devices to approach the equipment for transfer; accessible medical equipment (e.g., examination tables and chairs, mammography equipment, weight scales); patient lifts and other methods for transferring individuals from their mobility devices to medical equipment; and training health care personnel. In July 2010, DOJ also issued an advance notice of proposed rulemaking (ANPRM) announcing that, because of the obligation that has always existed under the ADA for covered entities to provide accessible equipment and furniture, DOJ was considering amending its regulations implementing Titles II and III of the ADA to include specific standards for the design and use of accessible equipment and furniture that is not fixed or built into a facility. These changes would ensure that programs and services provided by state and local governments and by public accommodations are accessible to individuals with disabilities (see 75 FR 43452, July 26, 2010).
Notes
A As for persons without disabilities, individual patients with disabilities have their own set of health conditions, including coexisting diseases and health risk factors that might affect the cost-benefit equation of obtaining various health care services. For example, although the U.S. Preventive Services Task Force gives colonoscopy screening to detect colorectal cancers a Grade A (service recommended: high certainty that the net benefit of the service is substantial), individual patients might determine in consultation with their physicians that the clinical risks of the bowel cleansing process required before colonoscopy outweigh the potential benefit of the test in their particular case. This decision should be based on clinical considerations and informed patients’ preferences, not on the inability to accommodate the needs of persons with disabilities during the bowel preparation.
B Secondary disabilities are conditions or complications that are related to a person’s primary disability and are also potentially disabling. Examples include injuries from falls, pressure ulcers, urinary tract complications, and depression.
C Each year, the National Healthcare Disparities Reports look at different measures of disparities, such as different types of service use or different measures of patients’ experiences with care.
D The National Health Interview Survey is a continuous federal survey overseen by the National Center for Health Statistics within the Centers for Disease Control and Prevention (CDC). Over the years, NHIS has been a major source of information about health care disparities for persons with disabilities among other vulnerable populations.
E Although the U.S. Preventive Services Task Force recommends mammography screening (for women ages 50-74 years old) and Pap smears (for women < age 65 who have been sexually active and have a cervix) with Grade B and Grade A evidence, respectively, these recommendations relate to broad populations of women. For each specific woman, the choices about whether to receive these screening services must be assessed based on her individual circumstances. For instance, women with severe, coexisting health conditions and high health risks may decide, in consultation with their physicians, that they will not benefit from this screening and choose not to have the tests.
F The clinical breast exam requires clinicians to palpate the entire breast, its perimeter, and immediately adjacent areas including the axilla (e.g., checking for lymph nodes). The breast tissue must be spread evenly over the chest wall, which requires women to be supine. With the woman lying flat on her back on an examination table, positioning her arm toward her head and rotating her hip and torso can assist in spreading the breast tissue.
G (1) “Are you limited in any way in any activities because of a physical, mental, or emotional problem?” (2) “Do you now have any health problems that require you to use special equipment such as a cane, a wheelchair, a special bed, or a special telephone?” and (3) “Do you consider yourself a person with a disability?”23 Those classified as having a disability were then asked about whether their disability was physical, sensory, mental, or learning (they could report more than one type) and whether their disability was slight, moderate, or severe (based on their own perceptions).
H (1) Experiencing restricted social activity. (2) Not knowing where to obtain disability resource information. (3) Needing home modifications but not having them. (4) Having difficulty accessing a health care provider’s office because of its physical layout or location. (5) being treated unfairly at a health care provider’s office.23
I The methods for measuring “low heights” for current height adjustable products vary among manufacturers and product designs. No official measurement method currently exists. Sections 4.1.2 and 5.1.4 describe the new standardized measurement method proposed by the Advisory Committee. Heights referenced in this report utilize this proposed new measurement method.
J As noted in Sections 1 and 8, this report does not address accessibility standards for children or for individuals with extreme obesity.
K Association for the Advancement of Medical Instrumentation. ANSI/AAMI ES60601-1:2005 (R) 2012. Medical Electrical Equipment—Part 1: General Requirements for Basic Safety and Essential Performance. Approved 9 February 2006 by American National Standards Institute, Inc. Revised 2012.
L Tilley, Alvin R. The Measure of Man and Woman: Human Factors in Design. New York: Wiley, 2002.
M U.S. Department of Labor. OSHA Technical Manual, Section VII, Chapter 1, Back Disorders and Injuries. OSHA website: http://www.osha.gov/dts/osta/otm/otm_vii/otm_vii_1.html (visited May 17, 2012).
N U.S. Department of Labor. Hospital eTool: Healthcare Wide Hazards – Ergonomics. OSHA website: http://www.osha.gov/SLTC/etools/hospital/hazards/ergo/ergo.html (visited May 17, 2012).
O Examples of settlements include Metzler v. Kaiser Permanente, Olson v. Sutter Health, University of California San Francisco Medical Center. Full settlement agreements are available online: http://thebarrierfreehealthcareinitiative.org/?page_id=16
P This list of consensus standards recognized by the FDA is available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfstandards/search.cfm
Q Information about the human factors design principles for medical devices is available at: http://www.aami.org/he75
R Western University of Health Sciences, University of California - San Francisco/Berkeley, University of Connecticut, University of Wisconsin-Milwaukee
Section 2: References
23. Centers for Disease Control and Prevention. Environmental barriers to health care among persons with disabilities, Los Angeles county, California, 2002-2003. Morbidity and Mortality Weekly Report. 2006;55(48):1300-1303.
Section 2: References
1. U.S. Department of Health and Human Services. Healthy People 2010. Second Edition, Understanding and Improving Health and Objectives for Improving Health. Second Edition ed. Washington, D.C.: U.S. Government Printing Office; 2000.
2. U.S. Department of Health and Human Services. The Surgeon General's Call to Action to Improve the Health and Wellness of Persons with Disabilities. Washington, D.C.: Public Health Service, Office of the Surgeon General; 2005.
3. Agency for Healthcare Research and Quality. 2009 National Healthcare Disparities Report. Vol AHRQ Publication No. 10-0004. Rockville, MD: U.S. Department of Health and Human Services; 2010.
4. Agency for Healthcare Research and Quality. 2010 National Healthcare Disparities Report. Vol AHRQ Publication No. 11-0005. Rockville, MD: U.S. Department of Health and Human Services; 2011.
5. National Council on Disability. The Current State of Health Care for People with Disabilities. Washington, DC: National Council on Disability; 2009.
6. Altman B, Bernstein A. Disability and Health in the United States, 2001-2005. Hyattsville, MD: National Center for Health Statistics; 2008.
7. Andriacchi R. Primary care for persons with disabilities. the internal medicine perspective. Am J Phys Med Rehabil. 1997;76(3 Suppl):S17-20.
8. Iezzoni LI. Blocked. Health Aff (Millwood). 2008;27(1):203-209. doi: 10.1377/hlthaff.27.1.203.
9. Kirschner KL, Breslin ML, Iezzoni LI. Structural impairments that limit access to health care for patients with disabilities. JAMA. 2007;297(10):1121-1125.
10. Drainoni M, Lee-Hood E, Tobias C, Bachman SS, Andrew J, Maisels L. Cross-disability experience of barriers to health-care access: Consumer perspectives. Journal of Disability Policy Studies. 2006;17(2):101-115.
11. Iezzoni LI, Kilbridge K, Park ER. Physical access barriers to care for diagnosis and treatment of breast cancer among women with mobility impairments. Oncology Nursing Forum. 2010;37(6):711-717.
12. Iezzoni LI, Killeen MB, O'Day BL. Rural residents with disabilities confront substantial barriers to obtaining primary care. Health Serv Res. 2006;41(4 Pt 1):1258-1275.
13. Iezzoni LI, O'Day BL. More than Ramps. A Guide to Improving Health Care Quality and Access for People with Disabilities. New York: Oxford University Press; 2006.
14. Iezzoni LI, Park ER, Kilbridge K. Implications of mobility impairment on the diagnosis and treatment of breast cancer. Journal of Women's Health. 2011;20(1):45-52.
15. Kroll T, Jones GC, Kehn M, Neri MT. Barriers and strategies affecting the utilisation of primary preventive services for people with physical disabilities: A qualitative inquiry. Health Soc Care Community. 2006;14(4):284-293.
16. Lishner DM, Richardson M, Levine P, Patrick D. Access to primary health care among persons with disabilities in rural areas: A summary of the literature. J Rural Health. 1996;12(1):45-53.
17. Mele N, Archer J, Pusch BD. Access to breast cancer screening services for women with disabilities. J Obstet Gynecol Neonatal Nurs. 2005;34(4):453-464.
18. Morrison EH, George V, Mosqueda L. Primary care for adults with physical disabilities: Perceptions from consumer and provider focus groups. Fam Med. 2008;40(9):645-651.
19. Scheer JM, Kroll T, Neri MT, Beatty P. Access barriers for persons with disabilities: The consumer's perspective. J Disabil Policy Stud. 2003;14(4):221-230.
20. Smeltzer SC, Sharts-Hopko NC, Ott BB, Zimmerman V, Duffin J. Perspectives of women with disabilities on reaching those who are hard to reach. J Neurosci Nurs. 2007;39(3):163-171.
21. Story MF, Schwier E, Kailes JI. Perspectives of patients with disabilities on the accessibility of medical equipment: Examination tables, imaging equipment, medical chairs, and weight scales. Disabil Health J. 2009;2(4):169-179.e1.
22. Bachman SS, Vedrani M, Drainoni M, Tobias C, Maisels L. Provider perceptions of their capacity to offer accessible health care for people with disabilities. J Disabil Policy Stud. 2006;17(3):130-136.
23. Centers for Disease Control and Prevention. Environmental barriers to health care among persons with disabilities, Los Angeles county, California, 2002-2003. Morbidity and Mortality Weekly Report. 2006;55(48):1300-1303.
24. Mudrick NR, Breslin ML, Liang M, Yee S. Physical accessibility in primary health care settings: Results from california on-site reviews. Disabil Health J. 2012;5(3):159-167.
25. Lagu T, Hannon NS, Rothberg MB, et al. Access to subspecialty care for patients with mobility impairment: A survey. Ann Intern Med. 2013;158(6):441-446.
26. Story MF, Winters JM, Lemke MR, et al. Development of a method for evaluating accessibility of medical equipment for patients with disabilities. Appl Ergon. 2010;42(1):178-183.
27. Story MF, Kaile JI, MacDonald C. The ADA in action at health care facilities. Disabil Health J. 2010;3:245-252.
28. Representative John Conyers Jr. (D-MI14). Nurse and health care worker protection act of 2009. 2009;H.R. 2381 (111th).
29. American Nurses Association. AMA leads initiative to develop national safe patient handling standards. multidisciplinary group seeks to establish evidence based guidelines to address deficiency. http://nursingworld.org/MainMenuCategories/WorkplaceSafety/Healthy-Work-Environment/SafePatient/ANA-Leads-National-Safe-Patient-Handling-Standards.pdf. Accessed May 31, 2013.
30. Bureau of Labor Statistics, U.S. Department of Labor. News release: Nonfatal occupational injuries and illnesses requiring days away from work, 2011. November 8, 2012;USDL-12-2204.
31. American Nurses Association, Nursing World. Saftey patient handling and mobility, health and safety. http://nursingworld.org/handlewithcare. Accessed April 27, 2012.
32. American Nurses Association, Nursing World. Safe patient handling and mobility (SPHM), state legislative agenda. http://nursingworld.org/MainMenuCategories/Policy-Advocacy/State/Legislative-Agenda-Reports/State-SafePatientHandling. Accessed April 27, 2012.
33. Waters TR, Putz-Anderson V, Garg A. Application Manual for the Revised NIOSH Lifting Equation. Pub. No. 94-110 ed. Cincinnati, OH: Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Division of Biomedical and Behavioral Science, DHHS (NIOSH); 1994.
34. Waters TR. When is it safe to manually life a patient?. AJN. 2007;107(8):53-58.
35. Story MF, Winters JM, Premo B, Kailes JI, Schwier E, Winters JM. Focus Groups on Accessibility of Medical Instrumentation. Atlanta, GA: Proceedings of RESNA 2005 Conference; 2005.
36. Winters JM, Story MF, Barnekow K, et al. Accessiblity of Medical Instrumentation: A Natilonal Healthcare Consumer Survey. Atlanta, GA: Proceedings of RESNA 2005 Conference; 2005.

User Comments/Questions
Add Comment/Question