Recommendations on Standards for the Design of Medical Diagnostic Equipment for Adults with Disabilities, Advisory Committee Final Report
4. Overview of Equipment Types
Different categories of medical diagnostic equipment have specific features or characteristics that affect their accessibility to individuals with disabilities and thus influence recommendations for ensuring access. Section 5 presents the detailed recommendations of the MDE Advisory Committee for MDE accessibility standards. Section 4 anticipates certain details of these recommendations by providing an overview of each of the five broad categories of MDE considered by the MDE Advisory Committee. These descriptions highlight specific attributes of each equipment type that the Advisory Committee considered in recommending accessibility standards.
4.1 Examination Tables and Chairs
4.1.1 Range of Examination Table and Chair Configurations
Examination tables and chairs are used wherever patients are examined throughout the health care delivery system. Therefore, tables and chairs must support a wide range of diagnostic activities, clinical indications, and patient populations. These demands have implications for the design, configuration, and principles of operation of examination tables and chairs. Broadly speaking, this equipment falls into two categories based on the positioning of the patient undergoing examination, those encompassed by the M301 and M302 criteria of the U.S. Access Board’s NPRM for MDE accessibility (see Section 1.3.1 and Tables 1.3.1(a) and 1.3.1(b)):
1. Equipment designed for patients in prone or side-lying positions (M301)
-
Examination tables
-
Podiatry tables
2. Equipment designed for patients seated or in semi-supine positions (M302)
-
Examination chairs
-
Dental chairs
-
Optometric chairs
-
Otolaryngology (ENT) chairs
-
Phlebotomy chairs
-
Podiatry chairs
As noted in the NPRM for MDE accessibility standards, when equipment is designed to support more than one patient position, the equipment would have to meet the technical criteria for each position supported. The word “prone” describes persons “lying on their stomachs” or “fronts” – in more technical language, in a ventral position. “Supine” indicates persons lying on their backs or with their faces facing upward. There are many positions between prone and supine, indicated by such terminology as semi-supine, Fowlers, semi-Fowlers, and semi-recumbent. For setting standards, these intermediate positions might be best considered in the M302 criteria. Some examination tables can be configured to have patients in either prone or seated positions (both M301 and M302 standards). Manufacturers design examination chairs that have, as their primary function, the support of patients in a seated or semi-supine position. While some chairs are capable of fully reclining, this is a secondary rather than primary functionality. In general, examination chairs are not designed for use in the prone or side-lying position.
4.1.2 Surface Design of Examination Tables and Chairs
For several reasons including patient comfort and safety, the surfaces of many examination tables and chairs are not flat. Instead, they are contoured, such as by using bolsters along the perimeters, to provide greater security once patients are lying or seated on the table or chair. This contouring complicates efforts to measure the distance between the floor and the height of the surface onto which patients transfer for their examinations. Thus, contouring must be addressed in considering minimum height standards for accessibility of transfer surfaces (Section 5.1.4).
As noted in Section 2.4.1, the height measurement method recommended by the Advisory Committee (Section 5.1.4) differs from the heights that manufacturers publish today. Currently, the de facto industry practice is to publish table or chair heights as measured while patients are seated on the equipment (i.e., while patients’ weights are compressing the table’s or chair’s foam padding).Z This dimension is typically measured at the back of a patient’s knees and includes compression of the seat foam. This method is consistent with the internationally recognized standard for the measurement of wheelchairs, ISO 7176-7:1998.
In contrast, the Advisory Committee recommends that measurement be taken from the highest point of the transfer surface, irrespective of contours, and with uncompressed foam (Section 5.1.4). Committee members recommend this measurement method because it ensures that the entire transfer surface is below the recommended height. This allows patients to choose the transfer location and technique that best meets their individual needs. For consistency, all transfer surface height measurements referenced in this report use the measurement method specified in Section 5.1.4, unless otherwise noted.
Notes
Z Seated height became the de facto height measurement method because this height is most relevant to clinicians as they conduct physical examinations of patients. Consequentially, health care professionals who are purchasing examination tables and chairs typically focus on the seated height dimension.
4.1.3 Adjustable Height Chairs with and without Footplates
Patients seated in adjustable height examination chairs typically rest their feet on the floor or on a footplate or have their feet hang with support from the lower portion of the chair. Because these chairs with footplates are adjustable in height, when the chair height is fully lowered, a minimum 2” clearance is required from the floor to the bottom of the footplate as specified by domestic UL and International Electrotechnical Commission (IEC) standards (as currently designed, footplates themselves are 1” thick at a minimum). For chairs without footplates, patients place their feet on the floor, but the retracted leg rest must maintain the same 2” clearance from the floor. Anthropometric data suggest the heel to popliteal dimension without shoes of males is 17.5” and of females is 15.9” at the 50th percentile. Current chair configuration and anthropometric factors inform the implications of minimum height standards for accessibility of chair transfer surfaces for future designs (Section 5.1.3).
The positioning of patients’ feet in relation to their seat height has critical implications for their comfort, as suggested in Figure 4.1. Contact with the back of the thigh and/or the rear of the knee with the top front portion of the seat is vital for patients’ comfort. This is especially important for maintaining the knee and thigh positions of individuals with disabilities who have difficulties controlling their lower extremities. If the distance between the footplate and the seat is too short (due to the required clearances described in the preceding paragraph), patients with disabilities could be uncomfortable during their diagnostic examinations and also have problems keeping their legs properly positioned.AA
For those chairs that transform into examination tables, reducing the distance between the floor and seat height may also restrict the overall length of the table when the patient is reclined into a supine position. Figure 4.1 shows the chair when configured in an upright seated position; these same patient support surfaces (made up of the back, seat, and leg rests) are repositioned horizontally to create a supine patient support surface. Therefore, a shorter leg rest results in an overall shorter horizontal table top. Some knee break chairs available in the market today have a total length of around 60”; with the addition of a flip-up or slide out footrest/leg rest extension, this total length increases to approximately 68”. Manufacturers expressed concern that reducing seat height will further reduce these table lengths, affecting the ability to adequately support patients in the supine position.
Figure 4.1
Anthropometric Data and Concept Drawing:
Support at Lower Thigh/Back of Knee for 17” and 19”
Distances between Footrest and Seat Heights
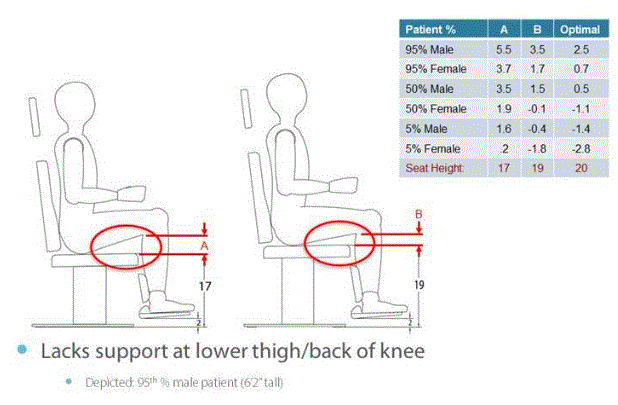
(SOURCE: Medical Technology Industries, Inc.)
Notes
AA For adjustable height chairs with footplates, as the height of the chair seat (transfer surface) moves up and down, the footplate moves up and down at the same time (i.e., with a fixed vertical distance between the seat and footplate). Therefore, reducing the low height of the seat will increase the likelihood of the footplate colliding with the floor. However, shortening the vertical distance between the seat and the footplate could result in the uncomfortable body positions shown in Figure 4.1.
4.2 Stretchers
Stretchers are ubiquitous throughout inpatient, outpatient surgery, and other health care settings. They are best known as transport devices for patients in supine, prone, or side-lying positions. However, as noted in Section 3.1, patients often undergo diagnostic evaluations while positioned supine, prone, or side-lying on a stretcher. This occurs especially in emergency departments. It is also common for patients to be transported within hospitals or other facilities for diagnostic procedures and to remain on the stretcher while the test is performed. The MDE Advisory Committee therefore included stretchers among MDE requiring consideration in setting accessibility standards.
Given their multipurpose usages, stretchers have various features that affect recommendations about their accessibility standards. Two features are especially important. First, to serve these multiple purposes, the current configuration of stretchers must accommodate the vertical height needs of numerous components, including (Figure 3.1): the basic elements of wheels, brakes, and steering systems; the green oxygen cylinders necessary to support patients on ventilators or who require supplemental oxygen; and assorted monitors and therapeutic devices, such as intravenous poles, infusion pumps, and Foley catheter bags. As currently designed, oxygen cylinders are typically transported in a horizontal position below the patient bed surface, consuming critical vertical height. Another component is the 6" high lift clearance window that forces foot operated controls up. With these essential features competing for the same vertical space, current stretchers cannot lower to the desired minimum height of 17" to 19" (see Sections 5 and 6). While current stretchers are height adjustable, they have a low deck of 20” to 23” without the surface (i.e., mattress) and would therefore not meet accessible minimum height standards.
The second feature is the patient support surface (mattress), which is currently purchased separately from the stretcher deck. The standard of care is to have a pressure-relieving surface on the stretcher to help prevent pressure ulcers. These patient support surfaces may range from 4” to 5” in thickness; these inches add to the vertical height of a stretcher when measuring from the floor to the top of the surface in its uncompressed state.
The Stretchers Subcommittee and full Advisory Committee discussed possibilities for stretcher redesign, emphasizing that current designs should not preclude future innovation. With respect to lowering stretchers, members discussed at least three ways to further reduce height: (1) allow the frame to lower into the lift clearance area when the stretcher is being used for an independent transfer; once the patient is on the mattress, the stretcher frame could be raised, permitting the stretcher to move normally; (2) use another type of oxygen cylinder or make the oxygen cylinder movable to allow the frame to lower to 17”; or (3) use a gel mattress that is not as thick as conventional mattresses.
Figure 4.2
Configuration of Features in Current Stretcher Design
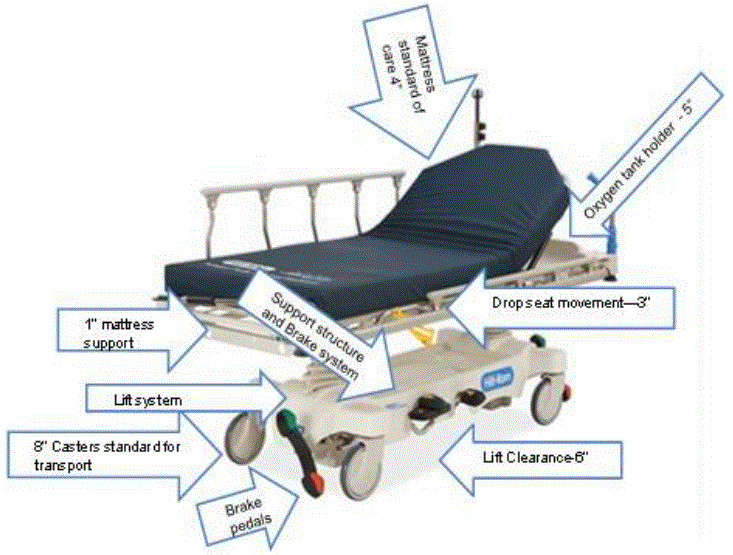
(SOURCE: Hill-Rom, Inc.)
MDE Advisory Committee members also discussed the possibility of developing different types of stretchers for different purposes. For example, stretchers used primarily in outpatient surgery centers for elective operations with patients who are not acutely ill might not require the oxygen cylinders or other equipment features that contribute to the vertical height of current stretchers. However, representatives from health care delivery systems argued that having different types of stretchers with different capabilities could reduce the efficiency of busy clinical settings. Research and development could allow future stretcher technologies to accommodate low-height accessibility standards (Section 5.1.3). But with today’s stretcher design, meeting those standards is infeasible given current requirements for multipurpose stretcher functions within health care delivery settings.
4.3 Diagnostic Imaging Equipment
Diagnostic imaging equipment encompasses a broad range of technologies comprised of integrated systems of components and accessories with diverse designs, configurations, and principles of operation (Table 4.1). This diversity reflects the wide range of diagnostic tasks, clinical indications, and patient populations that imaging equipment is designed to serve, both for common diagnostic needs (e.g., identifying broken bones) as well as for highly specialized clinical demands (e.g., evaluating blood flow in specific arteries, detecting tumor spread). The MDE Advisory Committee considered the full range of imaging equipment shown in Table 4.1, with initial discussions about accessibility standards occurring within the Diagnostic Imaging Subcommittee (Table 3.2) before full Committee consideration. Although it is a diagnostic imaging device, mammography equipment was addressed initially within its own Subcommittee before full Committee consideration; the issues raised by mammography are sufficiently different that they are described below (Section 4.3). As noted in Section 3.1, ultrasonography echocardiography equipment – technologically related diagnostic imaging modalities – are portable (i.e., configured within free-standing units typically positioned next to a table or stretcher upon which the patient lies during the test). Ultrasonography and echocardiography therefore do not fall under the scope of the proposed accessibility standards.
Table 4.3
Imaging Systems Considered by the MDE Advisory Committee
-
Computed tomography (CT)
-
Magnetic resonance (MR)
-
Single-photon emission computed tomography (SPECT)
-
Nuclear medicine (scintigraphy and single photon emission computed tomography) (NM)
-
Positron emission tomography (PET)
-
X-ray fluoroscopy
-
X-ray radiography
-
X-ray interventional
-
X-ray mobiles
-
X-ray C-arms
-
Dual-energy X-ray absorptiometry (DXA or DEXA)
-
X-ray mammography biopsy tables
-
PET/CT combined systems
-
NM/CT combined systems
-
PET/MR combined system
4.3.1 Overview of Imaging Equipment and Regulatory Environment
Diagnostic imaging equipment uses either ionizing radiation or a very strong magnetic field to produce the images used to diagnose a wide range of medical conditions, such as fractures, blood vessel blockages, various tissue abnormalities, and tumors. These machines typically involve a large capital outlay, last for many years of service, are operated by qualified technicians, and represent significant investments to health care facilities. Generally, this equipment is permanently mounted in a fixed installation within specially designed spaces that must perform specific essential functions, including supporting heavy weight, eliminating vibration, shielding ionizing radiation or magnetic fields, and having specialized high power capacity electrical service (see Section 7 for further description).
All testing using diagnostic imaging equipment is performed only under an order or prescription from a physician. Thus, before patients can have these tests, they must be evaluated by a physician, who then determines and requests the most appropriate diagnostic imaging test for the required evaluation. Diagnostic imaging equipment is operated only by trained and qualified technologists, who must be present during the examination. These technologists are present to assist patients, regardless of their physical abilities, onto the imaging equipment table and must ensure patients are properly positioned for the imaging procedure. Technologists also explain the purpose and necessary actions while they carry out the test. This equipment does not have patient “operable parts” – patients do not activate, deactivate, or adjust the imaging devices.
As described more fully in Sections 2.5 and 7.4, the U.S. Food and Drug Administration (FDA) regulates the majority of diagnostic imaging equipment as Class II medical devices, which need pre-market approval by FDA (510(k) clearance) prior to being placed on the market. The equipment must be designed and manufactured under the Quality System Regulations for medical devices, 21CFR820, which includes design controls and good manufacturing practices. An Occupational Safety and Health Administration (OSHA) credentialed Nationally Recognized Testing Laboratory must test and certify the equipment to demonstrate that it meets the basic safety and essential performance standards required by IEC 60601-1, as well as the applicable IEC 60601-1 series of collateral and particular standards. Devices that produce X-rays must also be certified by FDA as meeting the applicable performance standards for radiation safety found in 21CFRSubchapter J. The design process must include risk-management in accordance with ISO 14971. The Nuclear Regulatory Commission regulates the radioactive sources and radiopharmaceuticals used by nuclear medicine and PET equipment.
4.3.2 Transfer Surfaces and Imaging Equipment Functions
The transfer surface (table) of diagnostic imaging equipment serves two purposes: (1) it allows patients to be positioned properly to produce a high-quality image of the anatomical region of interest (with the lowest possible radiation dose for devices using radiation); and (2) imaging is conducted through the transfer surface. Thus, the transfer surface plays an integral role in the exam, is critical to achieving accurate diagnostic results, and influences radiation exposure of the patient. These requirements, combined with the diverse mechanical, electrical, and physics (e.g., functioning of magnets, electrical fields) aspects and needs of different diagnostic imaging equipment, generate a wide variety of equipment designs and configurations. These demands also place some inherent limitations on the design options for transfer surfaces or tables.
Diagnostic imaging equipment groups roughly into the following categories in terms of the functional role of the transfer surfaces, which has design implications:
-
Equipment with bores, including CT, PET, PET/CT, NM, and NM/CT. Here the table plays an integral part in achieving the sub-mm dynamic positioning accuracy needed during the scan.
-
Magnetic equipment that is open or has a bore. MR shares many similar aspects as equipment with bores, but has special considerations due to the very strong magnetic field.
-
DXA. In DXA, the X-ray source is positioned under the patient in a fixed, known geometry; this positioning maximizes diagnostic effectiveness and minimizes radiation doses (see below).
-
Conventional XR and fluoroscopy. This equipment has rectangular, radio-translucent tables that may translate in both directions in the horizontal plane.
-
Mobile XR. Mobile XR can be moved to the patient and can utilize detachable detectors that often can be placed behind the patient’s anatomy to be imaged without requiring significant movement by patients.
-
Interventional XR. This equipment, such as that used in cardiac catheterization/angiography suites, and “surgical” C-arms, virtually always involves patients who have been sedated to some extent prior to transfer onto the surface. After initial diagnostic evaluation (e.g., identification of location and degree of coronary artery blockages), often therapeutic interventions are performed (e.g., insertion of a stent through the catheter).
-
Prone breast biopsy tables. These devices are typically used for interventional procedures such as minimally invasive image guided biopsies. Its unique design must accommodate a physician underneath the patient support surface. Since the device is used in interventional procedures, patient sedation and/or local anesthetics are commonly used. These procedures are only performed after screening mammography and/or diagnostic mammography (or another diagnostic imaging assessment) has been performed. If a patient cannot access the prone table, another potential option is an upright stereotactic biopsy system, where patients are either seated or in a side-lying position.BB
Thus, diagnostic imaging equipment tables fall largely into two main groupings. The first group involves tables used by equipment with bores, such as CT, MR, and NM systems. These tables are generally long and relatively narrow in order to move the patient into the bore space. They are capable of adjusting with the high precision (sub-millimeter accuracy) needed for accurate diagnostic information both vertically and horizontally. These tables are typically rated for patients in excess of 400 lbs.
The second group is those tables used in X-Ray systems. These tables are also often rated for patients in excess of 400 lbs, but are wider than those used with equipment with bores and in many cases are able to move horizontally in two directions. Most current tables are not designed to adjust vertically, but some are capable of rotating to place the patient in a more vertical position needed for specific diagnostic exam.
As suggested by this technology and clinical overview and detailed further in Section 7.2, current structural requirements of specific imaging technologies have implications for the extent to which transfer surfaces on these devices can presently be height adjustable. Many X-Ray systems have imaging components such as X-Ray tubes, high voltage generators, and/or detectors located underneath the table (transfer surface). These components may, with current designs, impede lowering the table to accessible minimum heights.
An instructive example is the DXA scan (dual energy x-ray absorptiometry), used to measure bone density and identify persons with osteoporosis or osteopenia (low bone density but not yet osteoporosis). Bone loss is especially prevalent among persons with mobility disabilities who cannot perform weight-bearing exercise, such as walking or running. Therefore, access to DXA scans is critically important to a substantial subset of individuals with disabilities.
Typically, DXA scans measure bone density in the hip and spine as patients lie on a table and the scanning device – shaped like a large C with one arm passing above and the other passing immediately below the patient – travels from the hip area up to around the patient’s waist. This C-configuration, which brings the lower arm of the scanner close under the patient, minimizes the x-rays required to perform the test. Thus, the radiation exposure from DXA scans is low. However, to allow sufficient room to accommodate the x-ray technology in the lower arm of the C, the table on which patients must lie during DXA scans is relatively high off the ground. Current DXA technology cannot meet the recommended standards for height-adjustable tables with transfer surfaces at low heights.
As described further in Section 5, an issue closely related to transfer surfaces involves the positioning of supports to assist patient transfers. For diagnostic imaging equipment, patient support devices must meet applicable safety factors as delineated in IEC 60601-1. These factors typically range from 4 to 8 times the indicated weight support: for example, a transfer surface labeled to support a 500 lb patient must be designed and tested up to 4,000 lbs. This may have implications for adjustable height table design if, with current engineering methods, mechanical advantages (leverage) diminish as tables lower to lesser distances from the floor.
Notes
BB The Advisory Committee did not compare the relative effectiveness and safety of the prone breast biopsy table and the alternative upright stereotactic biopsy system to determine whether these are equivalent options for breast biopsies.
4.3.3 Imaging Equipment Used for Interventional and Biopsy Procedures with Sedated Patients
The MDE Advisory Committee viewed imaging systems used for both interventional radiology and biopsy procedures as raising special issues. Biopsies are explicitly diagnostic (i.e., biopsies provide tissue for pathological evaluation), even if they are followed by a procedure viewed as potentially therapeutic (e.g., an excisional biopsy, when an entire mass is removed). Interventional radiology includes such procedures as placement of stents in coronary arteries, opening narrowed blood vessels by dilating balloon catheters, and transarterial chemoembolization to block blood supplies to malignant tumors.
In most instances, patients undergoing these procedures receive some form of sedation prior to transfer.CC When sedated, all patients – regardless of their physical abilities – are assisted onto the transfer surface. For this reason, the Committee viewed imaging systems used for interventions and biopsies in patients who are typically sedated as outside the scope of the accessibility standards.
Notes
CC Patients are typically sedated to: minimize their discomfort during the procedure; and minimize their movements during procedures that often require very careful manipulation of instruments within constrained spaces (e.g., blood vessels). Patient movements in these circumstances could cause potentially life-threatening complications.
4.3.4 Industry Considerations in Designing Accessible Imaging Equipment
When considering applying accessibility standards to diagnostic imaging equipment, the MDE Advisory Committee recognized that certain constraints and performance requirements demand consideration, as follows:
-
Diagnostic accuracy must be equivalent across all patients;
-
Patients’ diagnostic needs and thus the clinical applications of the equipment vary widely;
-
Today’s technology has certain technical and diagnostic constraints, some of which relate to basic physics or physical properties of equipment elements (e.g., magnets, Section 7.2);
-
Equipment design must maintain accessibility for all patients and patient conditions.
-
Accessibility standards (e.g., support equipment) must preserve the physical access of technologists to the patient;
-
Equipment design must maintain infection control constraints;
-
Designs must adhere to FDA and international technical standards; and
-
A one-size-fits-all solution across equipment types is unlikely.
Today, it does not appear that any diagnostic imaging systems meet a minimum transfer height of 17” (see Sections 5 and 7). However, some current equipment with bore tables does lower to 18-19”. Redesign might allow some equipment (e.g., CT) to achieve a 17-19” minimum height standard. According to Committee members representing diagnostic imaging equipment manufacturers, certain aspects of redesign may take up to five years to engineer because of the technological complexities of particular diagnostic imaging systems. As described further in Section 7, most current diagnostic imaging technologies will encounter significant technical or diagnostic barriers to altering the actual transfer surface (table). Creative and alternative solutions are needed to facilitate independent transfers of individuals with disabilities.
As described in Section 7, one potential solution to improve accessibility of current imaging equipment designs are “system accessibility configurations” (referred to as “accessibility packages” during Advisory Committee discussions). However, some Committee members worried about aspects of suggested accessibility configurations, including potential safety hazards. Section 7 discusses these issues in further detail.
4.3.5 Patient Positions During Diagnostic Imaging
Finally, the vast majority of diagnostic imaging exams are conducted while patients are supine, prone, or side-lying on the table. Hence, the Committee focused much of its attention on the M301 standard (see Table 1.3.1(a)). However, given the enormous diversity of imaging equipment and the broad range of diagnostic objectives they seek to achieve, recommendations addressing M302 (diagnostic equipment used by patients in seated positions), M303 (diagnostic equipment used by patients seated in a wheelchair), and M304 (diagnostic equipment used by patients in standing position) are also relevant and were considered by the Committee.
Some nuclear medicine (NM) equipment has a unique design created for convenience: in this design, the table pivots out of the way to allow a scan of a patient seated in a chair or wheelchair. An experienced radiologist who presented to the Committee (see Section 3.3.3) indicated that in some circumstances examinations of equivalent quality may be obtained by patients who are supine on NM tables rather than sitting in chairs. Several Committee members and some radiologists expressed concerns that performing scans of patients while they remain in a wheelchair is not always appropriate and does not always produce images of equivalent quality as those obtained when patients are positioned properly on an imaging device. Therefore, the Committee used only the M301 criteria in making recommendations concerning NM accessibility standards.
Specialized MR and imaging devices using other modalities are designed specifically to scan upper and lower extremities (arms and legs). These specialized devices seat patients in chairs rather than positioning them on tables. The Committee agreed that, for these devices, the chair in which patients are seated should comply with the MDE accessibility standards recommended for chairs.
Certain X-ray exams are performed with patients asked to stand against a wall-like apparatus. In these situations, recommendations relating to M304 would apply (Section 5). Industry representatives on the Committee suggested that the standing supports would likely need to be an accessory or a mounting in the room. Additionally, these supports may also need to serve the diagnostic purpose of properly positioning the patient for the imaging tests. In these circumstances, recommendations relating to M305.3 may require adjustment to maintain diagnostic efficacy.
4.4 Mammography Equipment
According to figures from the CDC, breast cancer is the most common malignancy among women, although lung cancer causes the most cancer deaths in women (www.cdc.gov/cancer/dcpc/data/women.htm). Women with mobility disabilities are significantly less likely to undergo mammography screening for breast cancer than are other women, and as disability severity increases the rates of screening decrease (Table 2.1). Many factors contribute to these lower rates of screening mammography, but as noted in the NPRM (p. 40), inaccessibility of radiology equipment is a major reason persons with disabilities do not undergo imaging studies. As discussed further in Section 2.1, the U.S. Preventive Services Task Force recommends screening mammography every two years for women ages 50 through 74 (Grade B level of evidence). Depending on her specific clinical circumstances (e.g., comorbid illnesses, level of health risks), this recommendation should apply equally to many if not most women with disabilities.
Mammography is a specialized diagnostic imaging study that raises particular considerations. As noted in Section 4.3.1, technologists participate actively in all imaging studies. In the case of mammography, technologists provide hands-on help to all women, assisting them in positioning their bodies and breasts on the equipment’s breast plates to obtain the necessary images. Technologists report that conducting mammography studies of adequate quality is difficult when women remain seated in wheelchairs or scooters during the test. Depending on the configuration of the mammography machine, women seated in wheelchairs may not be able to get close enough to the equipment to position their breast as needed on the breast platform. In other instances, the breast platform might not go low enough to image their breasts while in the seated position.
Although for convenience throughout this section we refer to women as the users of mammography, men do – albeit rarely – undergo mammography. Generally men undergo mammography to evaluate breast enlargement, tenderness, or a palpable lump. Breast cancers occur in men, although rarely.DD Therefore, since women are the primary users of this equipment, the Committee focused on anthropometric measurements of women when possible.
Notes
DD According to the National Cancer Institute, less than 1% of breast cancers occur in men (http://www.cancer.gov/cancertopics/pdq/treatment/malebreast/Patient).
4.4.1 Overview of Mammography Accessibility Considerations
Mammography equipment will likely require redesign to fully accommodate women seated in wheelchairs. Critical considerations in this redesign include the physical constraints needed for operation of the equipment, technical feasibility, patient safety, clinical standards, and the range of body sizes, physical functional abilities, and wheelchair sizes.
Mammography imaging involves complex interactions between moving parts. Ordinarily, the accessibility dimensions are static and are measured from a static element, such as the floor. To get the final dimensions for the equipment standard, the subcommittee needed to consider the elements “dynamically,” especially given how the equipment operates. Recognizing that the movable breast platform is the main action point, recommendations define each component in relationship to the breast platform. The unique aspects of mammography device design and operation require components to work when the breast platform is at breast height. Since breast platform design affects most of the other device components, defining each component in relationship to the breast platform creates proportions that, when incorporated into the design process, assure that women seated in wheelchairs can get effective imaging.
Because of the movable breast platform and C-arm, each recommendation defines the other device components in relation to the breast platform. This gives the opportunity to consider and maximize the dimensions that improve the accessibility during the equipment’s operation. To make final recommendations for mammography accessibility, the Committee balanced the knowledge of disability, a range of physical characteristics and diversity of body types and sizes with each device component. Committee members used the best data available recognizing that the data available did not always match precisely the operations of mammography equipment.
4.4.2 Mammography Equipment and Scooters
The Committee considered the interaction of women who use scooters with mammography equipment. As noted above, to obtain acceptable images, a woman’s chest wall must be flush with the breast platform. However, the steering column and other front components of many scooters prevent this necessary proximity without the woman swiveling their seat to a side position. Once the scooter user swivels to the side, she may be able to be positioned on the equipment without any interference between her scooter and the equipment.EE The Committee’s mammography accessibility recommendations focus on interactions with wheelchair users. Mammography equipment recommendations also apply to use of a mammography chair for women who need to sit for the procedure but do not use a wheelchair and to women of short stature.
Notes
EE The ability of a scooter user to remain seated in her swiveled scooter seat and receive a successful mammogram image depends on multiple factors, including the size of her scooter, the ability to raise and lower the scooter seat, whether there is sufficient room to maneuver the scooter, her physical abilities (e.g., to rotate her torso), and the skill of the technologist.
4.4.3 Implications of Mammography Equipment Components
To determine the accessibility standard recommendations, understanding mammography equipment components is essential. Figure 4.3.3(a) illustrates the terminology and the location on a mammography device that the Committee used to describe mammography components.
Figure 4.4.3(a)
Mammography Equipment Components
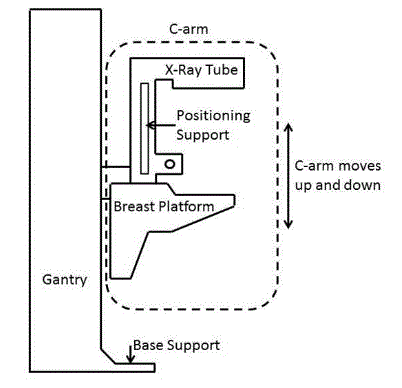
(SOURCE: Hologic, Inc.)
In order to get an image with sufficient detail, a woman’s breast must rest on top of the breast platform and her chest wall needs to be flush with the front edge of the breast platform. For this to be possible, the breast platform needs to go low enough to accommodate a woman seated in a wheelchair. In addition, knee and toe clearance must be adequate to allow the woman to get close enough to the breast platform without her knees or feet hitting parts of the equipment. Another important feature of mammography equipment is the base support (shown in Figure 4.4.3(a), which is critical for structural support, seismic stability, and installation safety. This base support must be low enough so that the woman’s wheelchair footrests can ride over it; furthermore, it must allow enough unobstructed floor space to ensure that her wheelchair’s front caster wheels do not hit it. Lastly, the configuration of the positioning supports must provide enough flexibility for all patients to be able to reach and hold them. Figure 4.4.3(b) shows an illustration of each accessibility feature.
Figure 4.4.3(b)
Features of Mammography Equipment Addressed by Committee
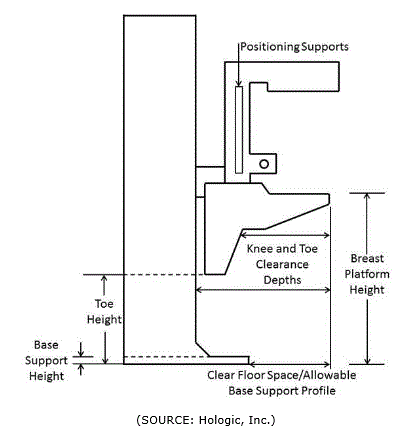
(SOURCE: Hologic, Inc.)
4.5 Weight Scales
Clinicians should periodically weigh all patients. Weight is a critical risk factor for many health conditions, including diabetes, cardiovascular diseases, arthritis, and certain cancers. For clinicians to counsel patients effectively about disease prevention, health promotion, and maximizing wellness, they therefore must know patients’ weights. In certain circumstances, such as setting chemotherapy dosages, weight is often a critical measurement. Without an accurate and current weight measurement, chances of missing diagnosis or incorrectly prescribing medications increase.
Obtaining an accurate weight is difficult if patients cannot stand on a scale without supports or assistance with balance. Wheelchair users, in particular, confront serious barriers to weight measurement. An accessible weight scale that can accommodate a wide range of patient populations and mobility devices solves that problem. Current scales with some accessible features come in various configurations, including wheelchair weight scales with single ramps, dual ramps, stand-on scales, and wall mounted scales with a folding platform. However, very few scales currently on the market are accessible to people who use wheelchairs or scooters.
The goal of the recommendations for weight scales is to accommodate the broadest range of individuals and mobility aid users. All standard size manual wheelchairs, powered wheelchairs, small to mid-range scooters, and most extra wide manual chairs can be accommodated through the Committee’s recommendations (see Section 5). However, some of the larger roadside scooter models, typically a 4-wheeled base scooter, may not fit onto the recommended platform size.
Sometimes scooter users are expected to be more mobile than wheelchair user and be able either to walk onto a scale or transfer to a stationary chair on a scale for weight measurement. However, not all scooter users can easily transfer from their scooters. Therefore, the Advisory Committee considered the likelihood that transfers for scooter users may be equally difficult as for individuals who use manual or power wheelchairs.
Alternative types of equipment that have integrated scales – such as examination tables, stretchers, portable lifts, and overhead ceiling lifts – can offer an effective means of taking a patient’s weight. Such equipment with integrated scales can eliminate the need for weighing the patient on a scale. If a patient transfers onto an examination table with an integrated scale, the clinician can also measure the patient’s weight as part of the physical exam. This would be convenient if the patient were already planning to get onto the examination table with an integrated scale. The medical diagnostic equipment accessibility standards that apply to exam tables and stretchers will also apply to such devices with integrated scales.
Notes
Z Seated height became the de facto height measurement method because this height is most relevant to clinicians as they conduct physical examinations of patients. Consequentially, health care professionals who are purchasing examination tables and chairs typically focus on the seated height dimension.
AA For adjustable height chairs with footplates, as the height of the chair seat (transfer surface) moves up and down, the footplate moves up and down at the same time (i.e., with a fixed vertical distance between the seat and footplate). Therefore, reducing the low height of the seat will increase the likelihood of the footplate colliding with the floor. However, shortening the vertical distance between the seat and the footplate could result in the uncomfortable body positions shown in Figure 4.1.
BB The Advisory Committee did not compare the relative effectiveness and safety of the prone breast biopsy table and the alternative upright stereotactic biopsy system to determine whether these are equivalent options for breast biopsies.
CC Patients are typically sedated to: minimize their discomfort during the procedure; and minimize their movements during procedures that often require very careful manipulation of instruments within constrained spaces (e.g., blood vessels). Patient movements in these circumstances could cause potentially life-threatening complications.
DD According to the National Cancer Institute, less than 1% of breast cancers occur in men (http://www.cancer.gov/cancertopics/pdq/treatment/malebreast/Patient).
EE The ability of a scooter user to remain seated in her swiveled scooter seat and receive a successful mammogram image depends on multiple factors, including the size of her scooter, the ability to raise and lower the scooter seat, whether there is sufficient room to maneuver the scooter, her physical abilities (e.g., to rotate her torso), and the skill of the technologist.

User Comments/Questions
Add Comment/Question